Abstract
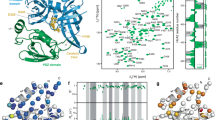
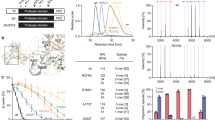
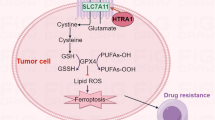
Serine protease activity of high temperature requrement 2 (HtrA2) is essential for promoting cell death, as well as for protecting against cellular stresses. An X-ray crystallographic study described the formation of a pyramid shaped homotrimer that is a proteolytically competent form of HtrA2; however, little is known about effects of the trimeric structure of HtrA2 on the natural substrates. In this study, we generated the HtrA2 protein that has a single point mutation at the homotrimerization motif to assess relationship between structure and the proteolytic activity of HtrA2 on its substrates. Using gel filtration, a native gel electrophoresis system, and a co-precipitation assay, we confirm that phenylalanine 149 in HtrA2 is a crucial determinant for the formation of the HtrA2 homotrimeric structure. Moreover, we described that the HtrA2 monomeric form abolished not only autoproteolytic activity, but also the proteolytic activity against XIAP (X-linked inhibitor of apoptosis protein) known as the HtrA2 substrate. Taken together, the results indicate that the homotrimeric structure of HtrA2 is required for executing its serine protease activity.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nam, MK., Seong, YM., Park, HJ. et al. The homotrimeric structure of HtrA2 is indispensable for executing its serine protease activity. Exp Mol Med 38, 36–43 (2006). https://doi.org/10.1038/emm.2006.5
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2006.5