Abstract
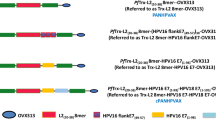
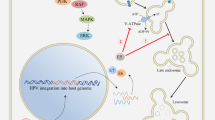
More than 99% of cervical cancers have been associated with human papillomaviruses (HPVs), particularly HPV type 16. The clear association between HPV infection and cervical cancer indicates that HPV serves as an ideal target for development of preventive and therapeutic vaccines. Although the recently licensed preventive HPV vaccine, Gardasil, has been shown to be safe and capable of generating significant protection against specific HPV types, it does not have therapeutic effect against established HPV infections and HPV-associated lesions. Two HPV oncogenic proteins, E6 and E7, are consistently co-expressed in HPV-expressing cervical cancers and are important in the induction and maintenance of cellular transformation. Therefore, immunotherapy targeting E6 and/or E7 proteins may provide an opportunity to prevent and treat HPV-associated cervical malignancies. It has been established that T cell-mediated immunity is one of the most crucial components to defend against HPV infections and HPV-associated lesions. Therefore, effective therapeutic HPV vaccines should generate strong E6/E7-specific T cell-mediated immune responses. DNA vaccines have emerged as an attractive approach for antigen-specific T cell-mediated immunotherapy to combat cancers. Intradermal administration of DNA vaccines via a gene gun represents an efficient way to deliver DNA vaccines into professional antigen-presenting cells in vivo. Professional antigen-presenting cells, such as dendritic cells, are the most effective cells for priming antigen-specific T cells. Using the gene gun delivery system, we tested several DNA vaccines that employ intracellular targeting strategies for enhancing MHC class I and class II presentation of encoded model antigen HPV-16 E7. Furthermore, we have developed a strategy to prolong the life of DCs to enhance DNA vaccine potency. More recently, we have developed a strategy to generate antigen-specific CD4+ T cell immune responses to further enhance DNA vaccine potency. The impressive pre- clinical data generated from our studies have led to several HPV DNA vaccine clinical trials.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hung, CF., Monie, A., Alvarez, R. et al. DNA vaccines for cervical cancer: from bench to bedside. Exp Mol Med 39, 679–689 (2007). https://doi.org/10.1038/emm.2007.74
Published:
Issue date:
DOI: https://doi.org/10.1038/emm.2007.74
Keywords
This article is cited by
-
A phase 1, single centre, open label, escalating dose study to assess the safety, tolerability and immunogenicity of a therapeutic human papillomavirus (HPV) DNA vaccine (AMV002) for HPV-associated head and neck cancer (HNC)
Cancer Immunology, Immunotherapy (2021)
-
Current status and future prospects for human papillomavirus vaccines
Archives of Pharmacal Research (2017)
-
Human papillomavirus: current status and issues of vaccination
Archives of Virology (2014)
-
Enhancing DNA vaccine potency by co-administration of xenogenic MHC class-I DNA
Gene Therapy (2010)