Abstract
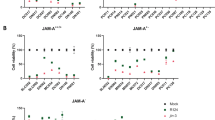
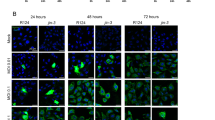
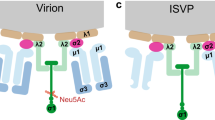
In the canonical pathway, infection of cells by the wild-type mammalian orthoreovirus Type 3 Dearing (T3D) is dependent on the interaction of the viral spike protein σ1 with the high-affinity cellular receptor junction adhesion molecule-A (JAM-A). We previously demonstrated that the human glioblastoma cell line U-118 MG does not express JAM-A and resists reovirus T3D infection in standard cell culture conditions (SCCC). Heterologous JAM-A expression sensitises U-118 MG cells to reovirus T3D. Here we studied reovirus infection in U-118 MG cells grown in spheroid cultures with the premise that cells in such cultures resemble cells in tumours more than those grown under standard adherent cell culture conditions on a plastic surface. Although the U-118 MG cells in spheroids do not express JAM-A, they are susceptible to reovirus T3D infection. We show that this can be attributed to factors secreted by cells in the spheroids. The concentration of active extracellular proteases cathepsin B and L in the medium of spheroid cultures was increased 19- and 24-fold, respectively, as compared with SCCC. These enzymes can convert the reovirus particles into a form that can infect the U-118 MG cells independent of JAM-A. Taken together, these data demonstrate that infection of tumour cells by wild-type reovirus T3D is not strictly dependent on the expression of JAM-A on the cell surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS . Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev 2010; 21: 91–98.
Duncan MR, Stanish SM, Cox DC . Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol 1978; 28: 444–449.
Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW . Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA 2004; 101: 11099–11104.
Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW . The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J 1998; 17: 3351–3362.
van Houdt WJ, Smakman N, van den Wollenberg DJ, Emmink BL, Veenendaal LM, van Diest PJ et al. Transient infection of freshly isolated human colorectal tumor cells by reovirus T3D intermediate subviral particles. Cancer Gene Ther 2008; 15: 284–292.
Alain T, Kim TS, Lun X, Liacini A, Schiff LA, Senger DL et al. Proteolytic disassembly is a critical determinant for reovirus oncolysis. Mol Ther 2007; 15: 1512–1521.
Mainou BA, Dermody TS . Transport to late endosomes is required for efficient reovirus infection. J Virol 2012; 86: 8346–8358.
Chappell JD, Duong JL, Wright BW, Dermody TS . Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J Virol 2000; 74: 8472–8479.
Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ et al. Junction adhesion molecule is a receptor for reovirus. Cell 2001; 104: 441–451.
Severson EA, Parkos CA . Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr Opin Cell Biol 2009; 21: 701–707.
Maginnis MS, Forrest JC, Kopecky-Bromberg SA, Dickeson SK, Santoro SA, Zutter MM et al. Beta1 integrin mediates internalization of mammalian reovirus. J Virol 2006; 80: 2760–2770.
Ebert DH, Deussing J, Peters C, Dermody TS . Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J Biol Chem 2002; 277: 24609–24617.
Danthi P, Guglielmi KM, Kirchner E, Mainou B, Stehle T, Dermody TS . From touchdown to transcription: the reovirus cell entry pathway. Curr Top Microbiol Immunol 2010; 343: 91–119.
Borsa J, Morash BD, Sargent MD, Copps TP, Lievaart PA, Szekely JG . Two modes of entry of reovirus particles into L cells. J Gen Virol 1979; 45: 161–170.
Bodkin DK, Nibert ML, Fields BN . Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol 1989; 63: 4676–4681.
Chappell JD, Barton ES, Smith TH, Baer GS, Duong DT, Nibert ML et al. Cleavage susceptibility of reovirus attachment protein sigma1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the sigma1 neck. J Virol 1998; 72: 8205–8213.
Golden JW, Schiff LA . Neutrophil elastase, an acid-independent serine protease, facilitates reovirus uncoating and infection in U937 promonocyte cells. Virol J 2005; 2: 48.
Nygaard RM, Golden JW, Schiff LA . Impact of host proteases on reovirus infection in the respiratory tract. J Virol 2012; 86: 1238–1243.
van den Wollenberg DJ, van den Hengel SK, Dautzenberg IJ, Cramer SJ, Kranenburg O, Hoeben RC . A strategy for genetic modification of the spike-encoding segment of human reovirus T3D for reovirus targeting. Gene Ther 2008; 15: 1567–1578.
Wilcox ME, Yang W, Senger D, Rewcastle NB, Morris DG, Brasher PM et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst 2001; 93: 903–912.
van den Hengel SK, Balvers RK, Dautzenberg IJ, van den Wollenberg DJ, Kloezeman JJ, Lamfers ML et al. Heterogeneous reovirus susceptibility in human glioblastoma stem-like cell cultures. Cancer Gene Therapy 2013; 20: 507–513.
Sutherland RM, Inch WR, McCredie JA, Kruuv J . A multi-component radiation survival curve using an in vitro tumour model. Int J Radiat Biol Relat Stud Phys Chem Med 1970; 18: 491–495.
Achilli TM, Meyer J, Morgan JR . Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 2012; 12: 1347–1360.
Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA . Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 2010; 148: 3–15.
De Witt Hamer PC, Van Tilborg AA, Eijk PP, Sminia P, Troost D, Van Noorden CJ et al. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene 2008; 27: 2091–2096.
Korff T, Augustin HG . Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 1998; 143: 1341–1352.
Mason SD, Joyce JA . Proteolytic networks in cancer. Trends Cell Biol 2011; 21: 228–237.
Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher ML, Mai J et al. Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Semin Cancer Biol 2005; 15: 149–157.
Antar AA, Konopka JL, Campbell JA, Henry RA, Perdigoto AL, Carter BD et al. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 2009; 5: 59–71.
Danthi P, Hansberger MW, Campbell JA, Forrest JC, Dermody TS . JAM-A-independent antibody-mediated uptake of reovirus into cells leads to apoptosis. J Virol 2006; 80: 1261–1270.
van den Wollenberg DJ, Dautzenberg IJ, van den Hengel SK, Cramer SJ, de Groot RJ, Hoeben RC . Isolation of reovirus T3D mutants capable of infecting human tumor cells independent of junction adhesion molecule-A. PLoS One 2012; 7: e48064.
Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res 2009; 15: 4374–4381.
Shmulevitz M, Gujar SA, Ahn DG, Mohamed A, Lee PW . Reovirus variants with mutations in genome segments S1 and L2 exhibit enhanced virion infectivity and superior oncolysis. J Virol 2012; 86: 7403–7413.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta 2012; 1824: 68–88.
Duffy MJ . The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des 2004; 10: 39–49.
Golden JW, Bahe JA, Lucas WT, Nibert ML, Schiff LA . Cathepsin S supports acid-independent infection by some reoviruses. J Biol Chem 2004; 279: 8547–8557.
Mainou BA, Dermody TS . In search of cathepsins: how reovirus enters host cells. DNA Cell Biol 2012; 31: 1646–1649.
Ho WJ, Pham EA, Kim JW, Ng CW, Kim JH, Kamei DT et al. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci 2010; 101: 2637–2643.
Wike-Hooley JL, Haveman J, Reinhold HS . The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol 1984; 2: 343–366.
Demchik LL, Sameni M, Nelson K, Mikkelsen T, Sloane BF . Cathepsin B and glioma invasion. Int J Dev Neurosci 1999; 17: 483–494.
Glimelius B, Norling B, Nederman T, Carlsson J . Extracellular matrices in multicellular spheroids of human glioma origin: increased incorporation of proteoglycans and fibronectin as compared to monolayer cultures. APMIS 1988; 96: 433–444.
Mok W, Boucher Y, Jain RK . Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res 2007; 67: 10664–10668.
Cheng J, Sauthoff H, Huang Y, Kutler DI, Bajwa S, Rom WN et al. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol Ther 2007; 15: 1982–1990.
Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther 1996; 7: 215–222.
Virgin HWt, Mann MA, Fields BN, Tyler KL . Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol 1991; 65: 6772–6781.
Faas FG, Avramut MC, van den Berg BM, Mommaas AM, Koster AJ, Ravelli RB . Virtual nanoscopy: generation of ultra-large high resolution electron microscopy maps. J Cell Biol 2012; 198: 457–469.
Doyle JD, Danthi P, Kendall EA, Ooms LS, Wetzel JD, Dermody TS . Molecular determinants of proteolytic disassembly of the reovirus outer capsid. J Biol Chem 2012; 287: 8029–8038.
Acknowledgements
We thank Dr Martine Lamfers and Anne Kleijn (Department of Neurosurgery, Erasmus MC, Rotterdam, The Netherlands) for help in generating spheroids on solid agarose matrices. This work was supported in part by the European Union through the 6th Framework Program GIANT (Contract No. 512087).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dautzenberg, I., van den Wollenberg, D., van den Hengel, S. et al. Mammalian orthoreovirus T3D infects U-118 MG cell spheroids independent of junction adhesion molecule-A. Gene Ther 21, 609–617 (2014). https://doi.org/10.1038/gt.2014.34
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/gt.2014.34
This article is cited by
-
Reovirus mutant jin-3 exhibits lytic and immune-stimulatory effects in preclinical human prostate cancer models
Cancer Gene Therapy (2022)
-
Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells
Scientific Reports (2017)
-
Replicating reoviruses with a transgene replacing the codons for the head domain of the viral spike
Gene Therapy (2015)