Summary
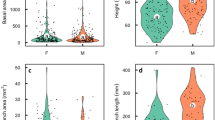
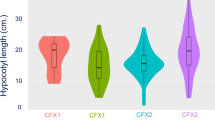
Sex ratios in 35 inflorescence and plant counts of perennial, sexually dimorphic Umbelliferae vary from 0·96 to 87·33 times as many males as females. The ranges of ratios are similar in dioecious and gynodioecious populations.
In 10 populations in which both the inflorescences and plants were counted, the male/female ratios are approximately one in populations in which the plants produce only one inflorescence per year and increase as the average number of inflorescences increases.
The interpretation offered is that the sex ratio is approximately one until reproduction begins, and in subsequent years increasingly male-biased ratios develop because sexual reproduction utilises more of the available resources of females than of males. Following reproduction, male plants survive longer and grow more and so become predominant.
In dioecious Angiosperms generally, male-biased ratios are characteristic of long-lived repeatedly flowering species and may be partly due to differential post-reproductive growth.
It is postulated that male preponderance is not directly selected for, but is a secondary consequence of separate competition among males and among females during sexual reproduction. The seed set and fitness of total populations may actually decrease with the development of marked male preponderance.
Similar content being viewed by others
Article PDF
References
Bliss, C I. 1967. Statistics in Biology, Vol. 1, McGraw-Hill, New York.
Bodmer, W F, and Edwards, A W F. 1960. Natural selection and the sex ratio. Ann Hum Genet, 24, 239–244.
Chater, A O, and Walters, S M. 1964. Silene. in Flora Europaea, Vol. 1, 158–181, ed. Tutin, T. G. et al. Cambridge University Press, Cambridge.
Coode, M J E, and Cullen, J. 1967. Silene. in Flora of Turkey and the East Aegean Islands, Vol. 2, 179–242, ed. Davis, P. H. Edinburgh University Press, Edinburgh.
Correns, C. 1928. Bestimmung, Vererbung und Verteilung des Geschlechtes bei den höheren Pflanzen. Handb Vererbungsw, 2, 1–138.
Dawson, J W. 1967. The New Zealand species of Gingidium. New Zealand J Bot, 5, 84–116.
Dawson, J W. 1971. Relationships of the New Zealand Umbelliferae. Bot J Linn Soc, 64, Supplement 1, 43–62.
Faegri, K, and Van Der Pijl, L. 1966. The Principles of Pollination Ecology. Pergamon Press Oxford.
Fisher, R A. 1930. The Genetical Theory of Natural Selection. Oxford University Press, Oxford.
Godley, E J. 1964. Breeding systems in New Zealand plants. 3. Sex ratios in some natural populations. New Zealand J Bot, 2, 205–212.
Harris, W. 1968. Environmental effects on the sex ratio of Rumex acetosella L. Proc New Zealand Ecol Soc, 15, 51–54.
Kalmus, H, and Smith, C A B. 1960. Evolutionary origin of sexual differentiation and the sex ratio. Nature, 186, 1004–1006.
Kaplan, S M. 1972. Seed production and sex ratio in anemophilous plants. Heredity, 28, 281–286.
Kolman, W. 1960. The mechanism of natural selection for the sex ratio. Amer Nat, 94, 373–377.
Lewis, D. 1941. Male sterility in natural populations of hermaphrodite plants. New Phytol, 40, 56–63.
Lewis, D. 1942. The evolution of sex in flowering plants. Cambr Phil Soc Biol Rev, 17, 46–67.
Lloyd, D G. 1972. Breeding systems in Cotula L. (Compositae, Anthemideae). 1. The array of monoclinous and diclinous systems. New Phytol, 71, 1181–1194.
Löve, A. 1943. Cytogenetic studies on Rumex subgenus Acetosella. Hereditas, 30, 1–136.
Martin, F W. 1967. Sex ratio and sex determination in Dioscorea. J Hered, 57, 95–99.
Mulcahy, D L. 1967. Optimal sex ratio in Silene alba. Heredity, 22, 411–423.
Murphy, G I. 1968. Pattern in life history and the environment. Amer Nat, 102, 391–404.
Putwain, P D, and Harper, J L. 1972. Studies in the dynamics of plant populations. V. Mechanisms governing the sex ratio in Rumex acetosa and R. acetosella. J Ecol, 60, 113–129.
Ross, M D. 1970. Evolution of dioecy from gynodioecy. Evolution, 24, 827–828.
Shore, B F. 1969. Dioecism in New Zealand Escalloniaceae. New Zealand J Bot, 7, 113–124.
Smith, B W. 1963. The mechanism of sex determination in Rumex hastatulus. Genetics, 48, 1265–1288.
Smith, B W. 1968. Cytogeography and cytotaxonomic relationships of Rumex paucifolius. Amer J Bot, 55, 673–683.
Sokal, R R, and Rohlf, F J. 1969. Biometry. Freeman, New York.
Wildish, D J. 1971. Adaptive significance of a biased sex ratio in Orchestia. Nature, 233, 54–55.
Zuk, J. 1963. An investigation on polyploidy and sex determination within the genus Rumex. Acta Soc Bot Pol, 32, 5–67.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lloyd, D. Sex ratios in sexually dimorphic umbelliferae. Heredity 31, 239–249 (1973). https://doi.org/10.1038/hdy.1973.79
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1973.79
This article is cited by
-
Spatial Segregation of the Sexes and Nutrients affect Reproductive Success in a Dioecious Wind-pollinated Grass
Plant Ecology (2005)
-
Sexual dimorphism, sex ratio and spatial distribution of male and female shrubs in the dioecious speciesPistacia lentiscus L.
Folia Geobotanica (1999)
-
Sex ratio in hybrids between Silene alba and Silene dioica: evidence for Y-linked restorers
Heredity (1994)
-
Sexual dimorphism and fruit production in a dioecious understory tree, Ilex opaca Ait.
Oecologia (1991)
-
Sex ratio variation and spatial segregation of the sexes in populations ofRumex acetosa andR. acetosella (Polygonaceae)
Plant Systematics and Evolution (1991)