Abstract
Several previous attempts at selecting for increased life span with Drosophila have failed to obtain a response to selection, and postulate that life span is controlled by non-genetic maternal effects instead of genes. In other experiments, however, populations have responded. This study uses a set of true-breeding long- and short-lived stocks developed by in vitro selection to examine the effect on life span of developmental environment and outcrossing.
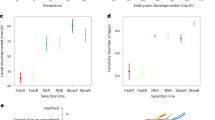
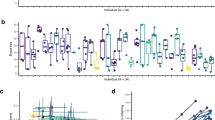
The expression of genes for life span is shown here to vary as a result of a gene-environment interaction and is strongly affected by the environment during development. The longevity of F1 crosses between long- and short-lived parental lines is additive when the density of larvae is high and unfixed, showing that life span is controlled by genes and not nongenetic maternal effects. But, when numbers of larvae are held low and constant, as in studies where selection fails, life span of crosses and parental lines is greatly restricted. The failure of some previous attempts at selection, therefore, appears to have resulted from the introduction, through experimental design, of strong artifactual environmental effects that limit the phenotypic expression of genes for life span and the effectiveness of selection.
Comparison of fecundity in parental and F1 lines shows that selection for increased life span antagonistically reduces early-life fecundity. Short-lived, parental lines, reproduced at an early age in life, lay 22–24 per cent more eggs in the same period than do long-lived lines selected for reproduction at a late age.
Similar content being viewed by others
Article PDF
References
Charlesworth, B. 1980. Evolution in Age-structured Populations. Cambridge University Press, Cambridge.
Derr, J A. 1980. The nature of variation in life history characters of Dysdercus bimaculatus (Heteroptera: Pyrrhocoridae), a colonizing species. Evolution, 34, 548–557.
Flanagan, J R. 1980. Detecting early-life components in the determination of the age of death. Mech Aging Dev, 13, 41–62.
Lansing, A I. 1947. A transmissible, cumulative and reversible factor in aging. J Gerontol, 2, 228–239.
Lansing, A I. 1954. A nongenetic factor in the longevity of rotifers. Ann N Y Acad Sci, 57, 455–464.
Lerner, I M. 1954. Genetic Homeostasis. Wiley, New York.
Lints, F A, and Gruwez, G. 1972. What determines the duration of development in Drosophila melanogaster. Mech Aging Dev, 1, 285–297.
Lints, F A, and Hoste, C. 1974. The Lansing Effect revisited. I. Life span. Exper Gerontol, 9, 51–69.
Lints, F A, and Hoste, C. 1977. The Lansing Effect revisited. II. Cumulative and spontaneously reversible effects on fecundity in Drosophila melanogaster. Evolution, 38(6), 996–1003.
Lints, F A, Stoll, J A, Gruwez, G, and Lints, C V. 1979. An attempt to select for increased longevity in Drosophila melanogaster. Gerontol, 5, 192–204.
Luckinbill, L S, Arking, R, Clare, M J, Cirocco, W, and Buck, S A. 1984. Selection for delayed senescence in Drosophila melanogaster. Evolution, 3(5), 996–1003.
Luckinbill, L S, Clare, M J. Selection for life span in Drosophila melanogaster. 1985. Heredity, 55, 9–18.
Medawar, P B. 1952. An Unsolved Problem in Biology. Lewis, London.
Murphy, P A, Giesel, J T, and Manlove, M N. 1983. Temperature effects on life history variation in Drosophila simulans. Evolution, 37, 1181–1191.
Parsons, P A. 1978. The genetics of aging in optimal and stressful environments. Exp Gerontol, 13, 357–363.
Parsons, P A. 1977. Genotype-temperature interactions for longevity in Drosophila simulans. Exp Gerontol, 12, 241–244.
Rose, M R. 1984. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution, 38(5), 1004–1009.
Rose, M R, and Charlesworth, B. 1980. A test of evolutionary theories of senescence. Nature, 287, 141–142.
Rose, M R, and Charlesworth, B. 1981. Genetics of life history in Drosophila melanogaster. II. Exploratory selection experiments. Genetics, 97, 187–196.
Waddington, C H. 1957. The strategy of the Genes. Allen and Unwin, London.
Waddington, C H. 1961. Genetic Assimilation. Adv Gen, 10, 257–293.
Williams, G C. 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution, 11, 398–411.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clare, M., Luckinbill, L. The effects of gene-environment interaction on the expression of longevity. Heredity 55, 19–26 (1985). https://doi.org/10.1038/hdy.1985.67
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1985.67
This article is cited by
-
Effects of telomere length in Drosophila melanogaster on life span, fecundity, and fertility
Chromosoma (2007)
-
Genetic approaches to study aging in Drosophila melanogaster
AGE (2005)
-
Genetic analysis of ageing: role of oxidative damage and environmental stresses
Nature Genetics (1996)
-
Inherited stress resistance and longevity: a stress theory of ageing
Heredity (1995)
-
Chromosomal localization and regulation of the longevity determinant genes in a selected strain of Drosophila melanogaster
Heredity (1993)