Abstract
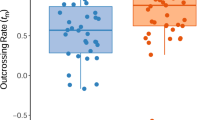
Measurements of mating patterns at the style morph and individual plant level within a population of tristylous Eichhornia paniculata from N.E. Brazil were made using allozyme and style morph marker loci. Application of a model of the mating system that incorporates the correlation of selling and of outcrossed paternal parentage indicated a high level of outcrossing at the population level (t̂=0·90±0·029). Parameter estimates from the model suggest that progeny from individual plants result from matings to a limited number of male parents. Thirty-two per cent of progeny within fruits were full sibs, compared to 24 per cent between fruits. Style morph segregation patterns in open-pollinated progenies indicated that most matings were between style morphs. Disassortative mating was significantly lower in the mid-styled morph in comparison with the long- and short-styled morphs. Significant variation in outcrossing rate among individuals was detected using two different multilocus procedures. Large statistical error associated with estimates of individual outcrossing rate will complicate attempts to correlate this variation with plant attributes.
Similar content being viewed by others
Article PDF
References
Barrett, S C H. 1985a. Ecological genetics of breakdown in tristyly. In Haeck, J. and Woldendorp, J. W. (ed.) Structure and Functioning of Plant Populations II Phenotypic and Genotypic Variation in Plant Populations, North-Holland Publishing Company, Amsterdam, pp. 267–275.
Barrett, S C H. 1985b. Floral trimorphism and monomorphism in continental and island populations of Eichhornia paniculata (Spreng.) Solms (Pontederiaceae). Biol J Linn Soc, 25, 41–60.
Barrett, S C H, and Glover, D. 1985. On the Darwinian hypothesis of the adaptive significance of tristyly. Evolution, 39, 766–774.
Barrett, S C H, and Shore, J. 1987. Variation and evolution of breeding systems in the Turnera ulmifolia complex (Turneraceae). Evolution, 41, 340–354.
Barrett, S C H, Brown, A H D, and Shore, J. 1987. Disassortative mating in tristylous Eichhornia paniculata (Pontederiaceae). Heredity, 58, 49–55.
Barrett, S C H, Morgan, M, and Husband, B. 1989. The Dissolution of A Complex Genetic Polymorphism: The Evolution of Self-Fertilization in Tristylous Eichhornia Paniculata. Evolution, 43, 1398–1416.
Brown, A H D. 1975. Sample sizes required to detect linkage disequilibrium between two or three loci Theoret. Popul Biol, 8, 184–201.
Brown, A H D. 1983. Multilocus Organization of Plant Populations. In Wohrmann, K. and Loeschcke, V. (Eds) Population Biology and Evolution, Springer-Verlag, Berlin, pp. 159–169.
Brown, A H D. 1989. Genetic characterization of plant mating systems. In Brown, A. H. D., Clegg, M. T., Kahler, A. L. and Weir, B. S. (eds) Plant Population Genetics, Breeding, and Genetic Resources, Sinauer Associates, Sunderland, Mass.
Brown, A H D, Barrett, S C H, and Moran, G. 1985. Mating system estimation in forest trees: models, methods and meanings. In Gregorius, H. R. (ed.) Population Genetics in Forestry, Springer-Verlag, Berlin, pp. 32–49.
Brown, A H D, Feldman, M, and Nevo, E. 1980. Multilocus structure of natural populations of Hordeum spontaneum. Genetics, 96, 523–536.
Brown, A H D, Grant, J, and Pullen, R. 1986. Outcrossing and paternity in Glycine argyrea by paired fruit analysis. Biol J Linn, Soc, 29, 283–294.
Chakraborty, R, Meagher, T, and Smouse, P. 1988. Parentage analysis with genetic markers in natural populations. I. The expected proportion of offspring with unambiguous paternity. Genetics, 118, 527–536.
Clegg, M. 1980. Measuring plant mating systems. Bioscience, 30, 814–818.
Crawford, T. 1984. What is a population? In Shorrocks, B. (ed.) Evolutionary Ecology, Blackwell Scientific Publications, Oxford, pp. 135–173.
Charlesworth, D, and Charlesworth, B. 1979. A model for the evolution of distyly. Amer Nat, 114, 467–498.
Crosby, J L. 1949. Selection of an unfavourable gene-complex. Evolution, 3, 212–230.
Darwin, C. 1877. The Different Forms of Flowers on Plants of the Same Species. John Murray, London.
Devlin, B, Roeder, K, and Ellstrand, N C. 1989. Fractional paternity assignment: theoretical development and comparison to other methods. Theoret Appl Genet, 76, 369–380.
Ellstrand, N C. 1984. Multiple paternity within the fruits of the wild radish, Raphanus sativus. Amer Nat, 123, 819–828.
Ellstrand, N C, Torres, A, and Levin, D. 1978. Density and the rate of apparent outcrossing in Helianthus annuus (Asteraceae). Syst Bot, 3, 403–407.
Ellstrand, N C, and Foster, K W. 1983. Impact of Population Structure On The Apparent Outcrossing Rate of Grain Sorghum (Sorghum Bicolor). Theoret Appl Genet, 66, 323327.
Ennos, R, and Clegg, M T. 1982. Effect of population substructuring on estimates of outcrossing rate in plant populations. Heredity, 48, 283–292.
Fyfe, J, and Bailey, N T J. 1951. Plant breeding studies in leguminous forage crops. I. Natural cross-breeding in winter beans. J Agric Sei, 41, 371–378.
Ganders, F. 1974. Disassortative Pollination in The Distylous Plant Jepsonia Heterandra. Canad J Bot, 52, 2401–2406.
Glover, D, and Barrett, S C H. 1986. Variation in The Mating System of Eichhornia Paniculata (Spreng.) Solms (Pontederiaceae). Evolution, 40, 1122–1131.
Glover, D, and Barrett, S C H. 1987. Genetic variation in continental and island populations of Eichhornia paniculata (Pontederiaceae). Heredity, 59, 7–17.
Hill, W. 1974. Estimation of linkage disequilibrium in randomly mating populations. Heredity, 33, 229–239.
Humphreys, M, and Gale, J. 1974. Variation in wild populations of Papaver dubium. VIII. The mating system. Heredity, 33, 33–41.
Levin, D, and Kerster, H. 1969. The dependence of bee-mediated pollen and gene dispersal upon plant density. Evolution, 23, 560–571.
Lloyd, D, and Webb, C. 1990. The evolution of heterostyly. In Nicholls, M. (ed.) Evolution and Function of Heterostyly, Springer-Verlag, Berlin, (in press).
Marshall, D, and Abbott, R. 1984. Polymorphism for outcrossing frequency at the ray floret locus in Senecio vulgaris L. III. Causes. Heredity, 53, 145–149.
Meagher, C. 1986. Analysis of paternity within a natural population of Chamaelirium luteum. I. Identification of most-likely male parents. Amer Nat, 128, 199–215.
Morgan, M T, and Barrett, S C H. 1989. Reproductive correlates of mating system variation in Eichhornia paniculata (Spreng.) Solms (Pontederiaceae). J Evol Biol, 2, 183–203.
Müller Starck, G. 1982. Sexually asymmetric fertility selection and partial self-fertilization. II. Clonal gametic contributions to the offspring of a Scots Pine seed orchard. Silva Fennica, 16, 99–106.
Ritland, K. 1988. The genetic-mating structure of subdivided populations. II. Correlated mating models. Theoret Popul Biol, 34, 320–346.
Ritland, K. 1989. Correlated matings in the partial selfer Mimulus guttatus. Evolution, 43, 848–860.
Ritland, K, and Jain, S K. 1981. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity, 47, 35–52.
Ritland, K, and El-Kassaby, Y A. 1985. The nature of inbreeding in a seed orchard of Douglas fir as shown by an efficient multilocus model. Theoret Appl Genet, 71, 375–384.
Ritland, K, and Ganders, F. 1985. Variation in the mating system of Bidens menziessii (Asteraceae) in relation to population substructure. Heredity, 55, 235–244.
Schemske, D, and Lande, R. 1985. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution, 39, 41–52.
Schmitt, J. 1983. Density-Dependent Pollinator Foraging, Flowering Phenology, and Temporal Pollen Dispersal Patterns in Linanthus Bicolor. Evolution, 37, 1247–1257.
Schoen, D. 1985. Correlation between classes of mating events in two experimental plant populations. Heredity, 55, 381–385.
Schoen, D. 1988. Mating system estimation via the one pollen parent model with the progeny array as the unit of observation. Heredity, 60, 439–444.
Schoen, D, and Clegg, M T. 1984. Estimation of Mating System Parameters When Outcrossing Events Are Correlated. Proc Natl Acad Sei Usa, 81, 5258–5262.
Schoen, D, and Stewart, S. 1986. Variation in Male Reproductive Success in White Spruce. Evolution, 40, 11091120.
Schou, O. 1983. The distyly in Primula elatior (L.) Hill (Primulaceae), with a study of flowering phenology and pollen flow. Bot J Linn Soc, 86, 261–274.
Shaw, D, Kahler, A, and Allard, R. 1981. Multilocus Estimation of Mating System Parameters in Plant Populations. Proc Natl Acad Sei Usa, 78, 1298–1302.
Smyth, C, and Hamrick, J. 1987. Realized gene flow via pollen in artificial populations of musk thistle, Carduus nutans L. Evolution, 41, 613–619.
Thomson, J, and Plowright, R C. 1980. Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia, 46, 68–74.
Willson, M, and Burley, N. 1983. Mate Choice in Plants: Tactics, Mechanisms, and Consequences. Princeton University Press, Princeton.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morgan, M., Barrett, S. Outcrossing rates and correlated mating within a population of Eichhornia paniculata (Pontederiaceae). Heredity 64, 271–280 (1990). https://doi.org/10.1038/hdy.1990.33
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1990.33
This article is cited by
-
Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia
Heredity (2006)
-
The influence of floral display size on selfing rates in Mimulus ringens
Heredity (2004)
-
Mating systems and interfertility of swamp milkweed (Asclepias incarnata ssp. incarnata and ssp. pulchra)
Heredity (1999)
-
Outcrossing rates of individual Mimulus ringens genets are correlated with anther–stigma separation
Heredity (1997)
-
The influence of population density on outcrossing rates in Mimulus ringens
Heredity (1995)