Abstract
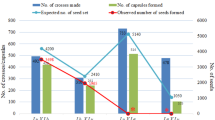
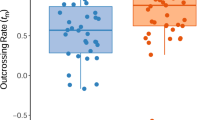
Experimental hand pollinations have revealed that the Australian proteaceous shrub Grevillea barklyana is fully self-compatible, although one study suggested that when both self- and outcross pollen were presented to different flowers on the same inflorescence significantly greater seed set resulted from the outcrossed flowers. This study used single-locus electrophoretic surveys of maternal plants and their progeny arrays to test the prediction that this apparent 'preference' for outcross pollen would produce high levels of outcrossing within natural populations. We found instead that plants within three of four populations were almost completely selfed. Outcrossing rates (t) in each of these populations (based on the progeny arrays of a minimum of nine plants) ranged from 0.07 ± 0.03 to only 0.33 ± 0.08 and showed little variation among years, ranging from 0.07 ± 0.03 to 0.10 ± 0.03 for a population sampled in each of two breeding seasons. Furthermore, examination of the progeny arrays from plants in the most intensively studied population revealed virtually no exchange of genes between immediately adjacent plants. Three pairs of alternative homozygotes were near neighbours (separated by less than 2 m) and yet detectable outcrosses comprised only seven of the 108 seeds sampled. In contrast, the fourth population of G. barklyana appeared highly outcrossed (t = 0.85 ± 0.2) which is typical of the realized mating system reported for other Australian Proteaceae. These data show that the realized mating system may vary widely among populations and may often be less than optimal. The occurrence of very low outcrossing rates within some populations may reflect the presence of introduced pollinators or other disturbances.
Similar content being viewed by others
Article PDF
References
Ayre, D J, and Whelan, R J. 1989. Factors controlling fruit set in hermaphroditic plants: studies with the Australian Proteaceae. Trends Ecol Evol, 4, 267–272.
Becerra, J X, and Lloyd, D G. 1992. Competition-dependent abscission of self-pollinated flowers of Phormium tenax (Agavavcea): a second action of self-incompatibility at the whole flower level? Evolution, 46, 458–469.
Brown, A H D, Barrett, S C H, and Moran, G F. 1985. Mating system estimation in forest trees: models, methods and meanings. In: H. R. Gregorius (ed.) Genetics in Forestry, pp. 32–49. Springer-Verlag, Berlin.
Brown, A H D, Matteson, A C, and Eldridge, K G. 1975. Estimation of mating system of Eucalyptus obliqua L'Herit. by using allozyme polymorphisms. Aust J Bot, 23, 931–949.
Carthew, S M. 1993. An assessment of pollinator visitation to Banksia spinulosa. Aust J Ecol (in press).
Carthew, S M, Ayre, D J, and Whelan, R J. 1988. High levels of outcrossing in populations of Banksia spinulosa R.Br, and Banksia paludosa Smith. Aust J Bot, 36, 217–223.
Charlesworth, D, and Charlesworth, B. 1987. Inbreeding depression and its evolutionary consequences. Ann Rev Ecol Syst, 18, 237–268.
Coates, D J, and Sokolowski, R E S. 1992. The mating system and patterns of genetic variation in Banksia cuneata A. S. George. Heredity, 69, 11–20.
Coldingay, R L, Carthew, S M, and Whelan, R J. 1991. The importance of non-flying mammals in pollination. Oikos, 61, 79–87.
Harris, H, and Hopkinson, D A. 1976. Handbook of Enzyme Electrophoresis in Human Genetics. North-Holland Publishing Co, Amsterdam.
Holtsford, T P, and Ellstrand, N C. 1992. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagaraceae). Evolution, 46, 216–225.
Hopper, S D, and Coates, D J. 1990. Conservation of genetic resources in Australia's flora and fauna. Proc Ecol Soc Aust, 16, 567–577.
Johnston, M O. 1992. Effects of cross and self-fertilization on progeny fitness in Lobelia cardinalis and L. siphilitica. Evolution, 46, 688–702.
Knight, S E, and Waller, D M. 1987. The genetic consequences of outcrossing in the cleistogamous annual, Impatiens capensis. I. Population genetic structure. Evolution, 41, 969–978.
McGillivray, D J. 1993. Grevillea: revision of the genus Grevillea. Melbourne University Press, Melbourne.
Montalvo, A M. 1992. Relative success of self and outcross pollen comparing mixed-and single-donor pollinations in Aquilegia caerulea. Evolution, 46, 1181–1188.
Schemske, D W, and Lande, R. 1985. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution, 39, 41–52.
Schoen, D J. 1982. The breeding system of Gilia achilleifolia: variation in floral characteristics and outcrossing rate. Evolution, 36, 596–613.
Scott, J K. 1981. Estimation of outcrossing rate for Banksia attenuata R.Br, and Banksia menziesii R.Br (Proteaceae) Aust J Bot, 28, 53–59.
Selander, R K, Smith, M H, Yang, Y S, Johnson, W B, and Gentry, J B. 1971. Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the old-field mouse (Peromyscus polionotus). Stud Gen Soc Austin, TX, 6, 49–90.
Shields, W M. 1982. Philopatry, Inbreeding and the Evolution of Sex. State University of New York Press, Albany.
Stephenson, A G, and Winsor, J A. 1986. Lotus corniculatus regulates offspring quality through selective fruit abortion. Evolution, 40, 453–458.
Soulé, M E. 1986. Conservation Biology. Sinauer, Sunderland.
Taylor, G, and Whelan, R J. 1988. Can honeybees pollinate Grevillea? Aust Zool, 24, 193–196.
Vaughton, G, and Carthew, S M. 1993. Evidence for selective abortion in Banksia spinulosa (Proteaceae). Biol J Lin Soc (in press).
Waser, N M, and Price, M V. 1989. Optimal outcrossing in Ipomopsis aggregata: seed set and offspring fitness. Evolution, 43, 1097–1109.
Waller, D M, and Knight, S E. 1989. Genetic consequences of outcrossing in the cleistogamous annual, Impatiens capensis. III. Interlocus associations. Heredity, 63, 1–9.
Willson, M F, and Burley, N. 1983. Mate Choice in Plants: Tactics, Mechanisms and Consequences. Princeton University Press, Princeton, NJ.
Wooller, R D, Russell, E M, Renfree, M B, and Towers, P A. 1983. A comparison of seasonal changes in the pollen loads of nectivorous marsupials and birds. Aust Wildl Res, 10, 311–317.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayre, D., Whelan, R. & Reid, A. Unexpectedly high levels of selfing in the Australian shrub Grevillea barklyana (Proteaceae). Heredity 72, 168–174 (1994). https://doi.org/10.1038/hdy.1994.24
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1994.24
Keywords
This article is cited by
-
Is the post-disturbance composition of a plant population determined by selection for outcrossed seedlings or by the composition of the seedbank?
Heredity (2014)
-
Integrating population demography, genetics and self-incompatibility in a viability assessment of the Wee Jasper Grevillea (Grevillea iaspicula McGill., Proteaceae)
Conservation Genetics (2008)
-
Genetic divergence among and diversity within two rare Banksia species and their common close relative in the subgenusIsostylis R.Br. (Proteaceae)
Conservation Genetics (2004)
-
Effects of seed bank disturbance on the fine-scale genetic structure of populations of the rare shrub Grevillea macleayana
Heredity (2003)
-
Genetic divergence and the mating system in the endangered and geographically restricted species, Lambertia orbifolia Gardner (Proteaceae)
Heredity (1999)