Abstract
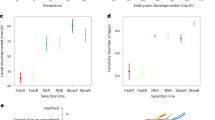
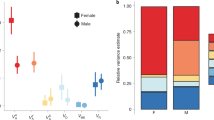
We measured the effect of larval density on thorax length, development time, sex ratio and a measure of total fitness, using strains of Drosophila melanogaster artificially selected for increased thorax length, control lines otherwise cultured in an identical way, and the base stock from which the lines had been derived. We used the addition experimental design (Mather & Caligari, 1981). No genotype–environment interaction was observed when comparing the reduction in thorax length of ‘large’ and ‘control’ lines with increasing larval density for any culture series, i.e. rank ordering of genotypes and additive genetic variances remained the same in all the environments tested. In contrast, the reduction in thorax length for the base stock as density increased was proportionally smaller than that of the ‘large’ and ‘control’ lines. Development time increased more rapidly with larval density in the ‘large’ lines than in the ‘controls’ or base stock. Sex ratio was unaffected by larval density but thorax length and the development time of females were more affected than those of males by increasing larval density. The estimate of total fitness showed clear evidence of gene–environment interaction for the effect of body size on fitness, with genetically large individuals at an increasing disadvantage with increasing larval density.
Similar content being viewed by others
Article PDF
References
Alpatov, W W. 1929. Growth and variation of the larvae of Drosophila melanogaster J Exp Zool, 42, 407–437.
Atkinson, W D. 1979. A field investigation of larval competition in domestic Drosophila. J Anim Ecol, 48, 91–102.
Atkinson, W D. 1985. Coexistence of Australian rain forest Diptera breeding in a fallen fruit. J Anim Ecol, 54, 507–518.
Ashburner, M, and Thompson, J N. 1978. The laboratory culture of Drosophila. In: Ashburner, M. and Wright, T. R. F. (eds) The Genetics and Biology of Drosophila, 2a, pp. 1–109. Academic Press, London.
Barker, K. 1959. Feeding period, growth and pupation in larvae of Drosophila melanogaster Ent Exp Appl, 2, 171–186.
Barker, K. 1961. An analysis of factors which determine success in competition for food among larvae of Drosophila melanogaster Arch Need Zool, 14, 200–281.
Bierbaum, T J, Mueller, L D, and Ayala, F J. 1989. Density-dependent evolution of life-history traits in Drosophila melanogaster. Evolution, 43, 382–392.
Botella, L M, Moya, A, Gonzalez, M C, and Mensua, J L. 1985. Larval stop, delayed development and survival in overcrowded cultures of Drosophila melanogaster. Effect of urea and uric acid. J Insect Physiol, 31, 179–185.
Budnik, M, and Brncic, D. 1976. Effects of larval biotic residues on viability of four species of Drosophila. Evolution, 29, 777–781.
Burnet, B, Sewell, D, and Bos, M. 1977. Genetic analysis of larval feeding behaviour in Drosophila melanogaster. II. Growth relations and competition between selected lines. Genet Res, 30, 149–161.
Caligari, P D S. 1980. Competitive interactions in Drosophila melanogaster. I. Monocultures. Heredity, 45, 219–231.
Church, R B, and Robertson, F W. 1966. Biochemical analysis of genetic differences in the growth of Drosophila melanogaster Genet Res, 1, 385–407.
Cole, L C. 1954. The population consequences of life history phenomena. Q Rev Biol, 19, 103–137.
Coyne, J A, and Beecham, E. 1987. Heritability of two morphological characters within and among natural populations of Drosophila melanogaster Genetics, 117, 727–737.
Dawood, M M, and Strickberger, M W. 1969. The effects of larval interaction on viability in Drosophila melanogaster. III. Effects of biotic residues. Genetics, 63, 213–220.
De Miranda, J R, and Eggleston, P. 1987. A comparison of substitution and addition design for the analysis of competitive interactions in Drosophila melanogaster. Heredity, 58, 279–288.
De Wit, C T. 1960. On competition. Versl Landbouwk Onderz Ned, 66, 1–82.
Gillespie, J H, and Turelli, M. 1989. Genotype-environment interactions and the maintenance of polygenic variation. Genetics, 121, 129–138.
Grimaldi, D, and Jaenike, J. 1984. Competition in natural populations of mycophagous Drosophila Ecology, 65, 1113–1120.
Lewontin, R C. 1965. Selection for colonizing ability. In: Baker, H. G. and Stebblins, G. L. (eds) The Genetics of Colonizing Species, pp. 79–94. Academic Press, New York.
Markow, T A. 1988. Reproductive behavior of Drosophila melanogaster and D. nigrospiracula in the field and in the laboratory. J Comp Psychol, 102, 169–173.
Mather, K, and Caligari, P D S. 1981. Competitive interactions in Drosophila melanogaster. II. Measurement of competition. Heredity, 46, 239–254.
Miller, R S. 1964. Larval competition in Drosophila melanogasterand D. simulans. Ecology, 45, 132–148.
Partridge, L, Ewing, A, and Chandler, A. 1987a. Male Size and mating success in Drosophila melanogaster. The roles of male and female behaviour. Anim Behav, 35, 555–562.
Partridge, L, and Farquhar, M. 1981. Sexual activity reduces lifespan of male fruitflies. Nature, 294, 580–582.
Partridge, L, and Farquhar, M. 1983. Lifetime mating success of male fruitflies (Drosophila melanogaster) is related to their size. Anim Behav, 31, 871–877.
Partridge, L, and Fowler, K. 1993. Responses and correlated responses to artificial selection on thorax length in Drosophila melanogaster. Evolution, 47, 213–226.
Partridge, L, Hoffman, A, and Jones, J S. 1987b. Male size and mating success in Drosophila melanogaster and Drosophila pseudoobscura under field conditions. Anim Behav, 35, 468–476.
Prout, T, and Barker, J S F. 1989. Ecological aspects of the heritability of body size in Drosophila buzzatii. Genetics, 123, 803–813.
Riska, B, Prout, T, and Turelli, M. 1989. Laboratory estimates of heritabilities and genetic correlations in nature. Genetics, 123, 865–871.
Robertson, F W. 1957. Studies in quantitative heritance. XI. Genetic and environment correlation between body size and egg production in Drosophila melanogaster. J Genet, 55, 428–443.
Robertson, F W. 1963. The ecological genetics of growth in Drosophila. 6. The genetic correlation between the duration of the larval period and body size in relation to larval diet. Genet Res, 4, 74–92.
Ruiz, A, Santos, M, Barbadilla, A, Quezada-Diaz, J E, Hasson, E, and Fontdevila, A. 1991. Genetic variance for body size in a natural population of Drosophila buzzatii. Genetics, 128, 739–750.
Sang, J H. 1949. The ecological determinants of population growth in a Drosophila culture. III. Larval and pupal survival. Physiol Zool, 22, 183–202.
Santos, M, Fowler, K, and Partridge, L. 1992a. On the use of tester stocks to predict the competitive ability of genotypes. Heredity, 69, 489–495.
Santos, M, Ruiz, A, Barbadilla, A, Quezada-Diaz, J E, Hasson, E, and Fontdevila, A. 1988. The evolutionary history of Drosophila buzzatii. XIV. Larger flies mate more often in nature. Heredity, 61, 255–262.
Santos, M, Ruiz, A, Quezada-Diaz, J E, Barbadilla, A, and Fontdevila, A. 1992b. The evolutionary history of Drosphila buzzatii. XX. Positive phenotypic covariance between field adult fitness components and body size. J Evol Biol, 5, 403–422.
Sewell, D, Burnet, B, and Connolly, K. 1975. Genetic analysis of larval feeding behaviour in Drosophila melanogaster. Genet Res, 24, 163–173.
Tantaway, A O, and Rakha, F A. 1964. Studies on natural populations of Drosophila. IV. Genetic variances of and correlations between four characters in D. melanogaster and D. simulans. Genetics, 65, 121–132.
Tantaway, A O, and Vetukhiv, M O. 1960. Effects of size on fecundity, longevity and viability in populations of Drosophila pseudoobscura. Am Nat, 94, 395–403.
Taylor, C E, and Kekic, V. 1988. Sexual selection in a natural population of Drosophila melanogaster. Evolution, 42, 197–199.
Weisbrot, D R. 1966. Genotypic interaction among competing strains and species of Drosophila. Genetics, 53, 427–435.
Wilkinson, G S. 1987. Equilibrium analysis of sexual selection in Drosophila melanogaster. Evolution, 41, 11–21.
Wilkinson, G S, Fowler, K, and Partridge, L. 1990. Resistance of genetic correlation structure to directional selection in Drosophila melanogaster. Evolution, 44, 1990–2003.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Santos, M., Fowler, K. & Partridge, L. Gene–environment interaction for body size and larval density in Drosophila melanogaster: an investigation of effects on development time, thorax length and adult sex ratio. Heredity 72, 515–521 (1994). https://doi.org/10.1038/hdy.1994.69
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1994.69
Keywords
This article is cited by
-
Evaluating the genetic architecture of quantitative traits via selection followed by inbreeding
Heredity (2019)
-
Artificial selection of insects to bioconvert pre-consumer organic wastes. A review
Agronomy for Sustainable Development (2019)
-
Effect of Toxic Heavy Metal Containing Industrial Effluent on Selected Life History Traits, Adult Morphology and Global Protein Expression Pattern of Drosophila melanogaster
Proceedings of the Zoological Society (2018)
-
Size relationships of different body parts in the three dipteran species Drosophila melanogaster, Ceratitis capitata and Musca domestica
Development Genes and Evolution (2016)
-
Plastic Responses to Temperature Versus Local Adaptation at the Cold Extreme of the Climate Gradient
Evolutionary Biology (2015)