Abstract
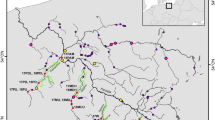
Using horizontal agarose thin layer gel electrophoresis, 35 allozyme loci were screened in 233 brown trout (Salmo trutta L.) from 11 populations in southwest Germany across the Rhenanian-Danubian watershed. Polymorphism was found at 10 loci, with stocked populations exhibiting significantly increased polymorphism compared with unmanaged stocks (P = 0.219 vs. P = 0.132). Standard genetic distances between populations from different brooks averaged at D = 0.01. Of the total gene diversity of GST = 0.198, only a negligible amount partitioned between Rhenanian and Danubian drainages (GGT = 0.010). One biallelic locus, LDH-5*, indicated river-specific allele frequencies, with the allele LDH-5*105 being markedly more frequent within the Danubian drainage system. In contrast, LDH-5*100 was close to fixation in the Rhenanian populations. This locus suggests a phylogeographical relationship of Danubian trout from southwest Germany with brown trout from southeastern Europe rather than with conspecifics of adjacent Rhenanian origin.
Similar content being viewed by others
Article PDF
References
Aebersold, P B, Winans, G A, Teel, D J, Milner, G B, and Utter, F M. 1987. Manual for starch gel electrophoresis: a method for the detection of genetic variation. NOAA Technical Report NMFS 61.
Allendorf, F W, Mitchell, N, Ryman, N, and Stahl, G. 1977. Isozyme loci in brown trout (Salmo trutta L.): detection and interpretation from population data. Hereditas, 86, 179–190.
Allendorf, F W, Ryman, N, Stennek, A, and Stahl, G. 1976. Genetic variation in Scandinavian brown trout (Salmo trutta L.): evidence of distinct sympatric populations. Hereditas, 83, 73–82.
Balon, E K. 1968. Notes on the origin and evolution of trouts and salmon with special reference to the Danubian trouts. VěstčslzemědMus, 32, 1–21.
Barbat-Leterrier, A, Guyomard, R, and Krieg, F. 1989. Introgression between introduced domesticated strains and Mediterranean native populations of brown trout (Salmo trutta L.). Aquat Living Resour, 2, 215–223.
Bernatchez, L, Guyomard, R, and Bonhomme, F. 1992. DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout populations. Mol Ecol, 1, 161–173.
Borcherdt, C. 1991. Bundesrepublik Deutschland 5 Baden-Württemberg: eine geographische Landeskunde. Wissenschaftliche Buchgesellschaft, Darmstadt.
Chakraborty, R, and Leimar, O. 1987. Genetic variation within a subdivided population. In: Ryman, N. and Utter, F. (eds) Population Generics and Fisheries Management pp. 89–120. University of Washington Press, Seattle and London.
Daye, P G, and Garside, E T. 1979. Development and survival of embryos and alevins of the Atlantic salmon, Salmo salar, continuously exposed to acidic levels of pH, from fertilization. Can J Zool, 57, 1713–1718.
Felsenstein, J. 1989. PHYLIP: Phylogeny Inference Package (version 3.2). Cladistics, 5, 164–166.
Ferguson, A. 1989. Genetic differences among brown trout, Salmo trutta, stocks and their importance for the conservation and management of the species. Freshwater Biol, 21, 35–46.
Garcia-Marin, J L, Jorde, P E, Ryman, N, Utter, F, and Pla, C. 1991. Management implications of genetic differentiation between native and hatchery populations of brown trout (Salmo trutta) in Spain. Aquaculture, 95, 235–249.
Guyomard, R, and Krieg, F. 1983. Electrophoretic variation in six populations of brown trout (Salmo trutta L.). Can J Genet Cytol, 25, 403–413.
Hamilton, K E, Ferguson, A, Taggart, J B, Tomasson, T, Walker, A, and Fahy, E. 1989. Post-glacial colonization of brown trout, Salmo trutta L.: Ldh-5 as a phylogeographic marker locus. J Fish Biol, 35, 651–664.
Hantke, R. 1993. Fluβgeschichte Mitteleuropas. Ferdinand Enke Verlag, Stuttgart.
Harris, H, and Hopkinson, D A. 1976. Handbook of Enzyme Electrophoresis in Human Genetics. American Elsevier, New York.
Hauser, L, Beaumont, A R, Marshall, G T H, and Wyatt, R J. 1991. Effects of sea trout stocking on the population genetics of landlocked brown trout, Salmo trutta L., in the Conwy River system, North Wales, U.K. J Fish Biol, 39, (Suppl. A), 109–116.
Henry, T, and Ferguson, A. 1985. Kinetic studies on the lactate dehydrogenase (LDH-5) isozymes of the brown trout, Salmo trutta L. Comp Biochem Physiol, 82B, 95–98.
Hindar, K, Ryman, N, and Utter, F. 1991. Genetic effects of cultured fish on natural populations. Can J Fish Aquat Sci, 48, 945–957.
Karakousis, Y, and Triantaphyllidis, C. 1990. Genetic structure and differentiation among Greek brown trout (Salmo trutta L.) populations. Heredity, 64, 297–304.
Krieg, F, and Guyomard, R. 1985. Population genetics of French brown trout (Salmo trutta L.): large geographical differentiation of wild populations and high similarity of domesticated stocks. Génét Sél Évol, 17, 225–242.
Lelek, A. 1988. Vorkommen, Taxonomie und Maßnahmen zur Erhaltung der Forelle Salmo trutta labrax Pallas 1811 in der NO-Türkei. Courier Forsch-Inst Senckenberg, 101, 1–44.
Mader, M. 1978. Die Flußgeschichte des Neckars und das Wandern des Albtraufs. Veröff Naturschutz Landschaftspflege Bad-Württ 47/48, 443–507.
Martinez, P, Arias, J, Castro, J, and Sanchez, L. 1993. Differential stocking incidence in brown trout (Salmo trutta) populations from Northwestern Spain. Aquaculture, 114, 203–216.
Møller Hansen, M, Loeschcke, V, Rasmussen, G, and Simonsen, V. 1993. Genetic differentiation among Danish brown trout (Salmo trutta) populations. Hereditas, 118, 177–185.
Moran, P, Pendas, A M, Garcia-Vazquez, E, and Izquierdo, J. 1991. Failure of a stocking policy, of hatchery reared brown trout, Salmo trutta L., in Asturias, Spain, detected using LDH-5* as a genetic marker. J Fish Biol, 39, 117–121.
Müller, H. 1956. Die Forellen Die einheimischen Forellen und ihre wirtschaftliche Bedeutung. Neue Brehmbücherei 164. A. Ziemsen Verlag, Wittenberg, Lutherstadt.
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590.
Nelson, K, and Soulé, M. 1987. Genetical conservation of exploited fishes. In: Ryman, N. AND Utter, F. (eds) Population Genetics and Fisheries Management pp. 345–368. University of Washington Press, Seattle and London.
Osinov, A G. 1984. Zoogeographical origins of brown trout, Salmo trutta (Salmonidae): data from biochemical genetic markers. J Ichthyol, 24, 10–23.
Ryman, N. 1983. Patterns of distribution of biochemical genetic variation in salmonids: differences between species. Aquaculture, 33, 1–21.
Ryman, N, Allendorf, E W, and Stahl, G. 1979. Reproductive isolation with little genetic divergence in sympatric populations of brown trout (Salmo trutta). Genetics, 92, 247–262.
Schaal, B A, and Anderson, W W. 1974. An outline of techniques for starch gel electrophoresis of enzymes from American oyster Crassostrea virginica Gmelin. Georgia Marine Science Center Technical Report 74–3.
Shaklee, J B, Allendorf, F W, Morizot, D C, and Whitt, G S. 1990. Gene nomenclature for protein-coding loci in fish. Trans Am Fish Soc, 119, 2–15.
Skaala, O. 1992. Genetic population structure of Norwegian brown trout. J Fish Biol, 41, 631–646.
Taggart, J B, and Ferguson, A. 1986. Electrophoretic evaluation of a supplemental stocking programme for brown trout, Salmo trutta L. Aquaculture Fish Manage, 17, 155–162.
Taggart, J, Ferguson, A, and Mason, F M. 1981. Genetic variation in Irish populations of brown trout (Salmo trutta L.): electrophoretic analysis of allozymes. Comp Biochem Physiol, 69B, 393–412.
Wagner, G. 1963. Danubische und rheinische Abtragung im Neckar- und Tauberland. Ber dt Landesk, 31, 1–11.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riffel, M., Storch, V. & Schreiber, A. Allozyme variability of brown trout (Salmo trutta L.) populations across the Rhenanian–Danubian watershed in southwest Germany. Heredity 74, 241–249 (1995). https://doi.org/10.1038/hdy.1995.37
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1995.37
Keywords
This article is cited by
-
Will the genomics revolution finally solve the Salmo systematics?
Hydrobiologia (2022)
-
Genetic variation in brown trout Salmo truttaacross the Danube, Rhine, and Elbe headwaters: a failure of the phylogeographic paradigm?
BMC Evolutionary Biology (2013)
-
Genetic Structure of Brown Trout (Salmo trutta) Populations from Turkey Based on Microsatellite Data
Biochemical Genetics (2010)
-
Differences in levels of heterozygosity in populations of the common gudgeon (Gobio gobio, Cyprinidae) among adjacent drainages in Central Europe: an effect of postglacial range dynamics?
Heredity (2002)
-
Postglacial colonization of brown trout in Europe based on distribution of allozyme variants
Heredity (1999)