Abstract
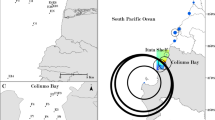
Bottleneck effects in three local populations of the fossorial rodent Ctenomys maulinus brunneus as caused by the recent eruption of the Lonquimay volcano in the Andes of Southcentral Chile are presented. Comparative census estimates in Río Colorado indicated a 91.3 per cent decrease in the breeding population size after the catastrophe. All parameters of genetic diversity were drastically affected and surpassed neutral expectations in each population. The proportion of polymorphic loci decreased by 57 per cent, 100 per cent and 83.2 per cent in the bottleneck populations of Río Colorado, Las Raíces and Alto Bío Bío, respectively. The source populations were estimated to have 2.2, 1.5 and 1.4 alleles per locus whereas the three derived populations had estimated values of 1.4, 1.0 and 1.1, respectively. Average heterozygosity dropped by 71 per cent, 100 per cent, and 57 per cent in the same populations, respectively. The spatial genetic structuring observed before the eruption indicated a high degree of population subdivision (Wahlund effect), consistent with an isolation-by-distance model. After the eruption, excessive microspatial genetic differentiation and larger-scale homogeneity indicated drastic disruption of the breeding or social units. Low levels of genetic variation in Andean Ctenomys, claimed to be an adaptive response to the stable subterranean niche, can be attained by the recurrent catastrophe-induced effects of genetic drift.
Similar content being viewed by others
Article PDF
References
Archie, J W. 1985. Statistical analysis of heterozygosity data: independent sample comparisons. Evolution, 39, 623–637.
Barrientos, S E, and Acevedo-Aranguiz, P S. 1992. Seis- mological aspects of the 1988–1989 Lonquimay (Chile) volcanic eruption. J Volcan Geothermal Res, 53, 73–87.
Baker, A J, and Moeed, A. 1987. Rapid genetic differentiation and founder effect in colonizing populations of common mynas (Acridotheres tristis). Evolution, 41, 525–538.
Barton, N H. 1989. Founder effect speciation. In: Otte, D. and Endler, J. A. (eds) Speciation and its Consequences, pp. 229–256, Sinauer Associates, Sunderland, MA.
Beaucournu, J C, and Gallardo, M H. 1977. Quelques nouvelles puces du Chili (Siphonaptera), parasites du Ctenomys (Rod., Octodontidae). Bull Soc Pathol Exotique, 70, 438–450.
Berry, R J. 1986. Genetics of insular populations of mammals, with particular reference to differentiation and founder effects in British small mammals. Biol J Linn Soc, 28, 205–230.
Bonnell, M L, and Selander, R K. 1974. Elephant seals: genetic variation and near extinction. Science, 184, 908–909.
Bryant, E H, and Mefert, L M. 1990. Multivariate phenotypic differentiation among bottleneck lines of the housefly. Evolution, 44, 660–668.
Carson, H L, and Templeton, A R. 1984. Genetic revolutions in relation to speciation phenomena: the founding of new populations. Ann Rev Ecol Syst, 15, 97–131.
Chakraborty, R, and Nei, M. 1977. Bottleneck effects on average heterozygosity and genetic distance with the stepwise mutation model. Evolution, 31, 347–356.
Chepko-Sade, B D, Shields, W M, Berger, J, Tang Halpin, Z, Thomas Jones, W, Rogers, L L, Rood, J P, and Smith, A T. 1987. The effects of dispersal and social structure on effective population size. In: Chepko-Sade, B. D. and Tang Halpin, Z. (eds) Mammalian Dispersal Patterns, pp. 287–321, The University of Chicago Press, Chicago, IL.
Chesser, R K, and Ryman, N. 1986. Inbreeding as a strategy in subdivided populations. Evolution, 40, 614–624.
Coates, D J. 1992. Genetic consequences of a bottleneck and spatial genetic structure in a triggerplant Stylidium coroniforme (Stylidiaceae). Heredity, 69, 512–520.
Dodd, D M B, and Powell, J R. 1985. Founder-flush speciation: an update of experimental results with Drosophila. Evolution, 39, 1388–1392.
Easteal, S. 1985. The ecological genetics of introduced populations of the giant toad Bufo marinus. II. Effective population size. Genetics, 110, 107–122.
Eguiarte, L E, Burquez, A, Rodriguez, J, Martinez-Ramos, M, Saruklan, J, and Pinero, D. 1993. Direct and indirect estimates of neighborhood and effective population size in a tropical palm, Astrocaryum mexicanum. Evolution, 47, 75–87.
Freiberg, H M. 1985. Vegetationskundliche Untersuchungen an Sudchilenischen Vulkanen. Bonn Geograph Abhandl, 70, 1–170.
Gallardo, M H. 1979. Las especies chilenas de Ctenomys. I. Estabilidad cariotípica. Arch Biol Med Exp, 12, 71–82.
Gallardo, M H. 1991. Karyotypic evolution in Ctenomys (Rodentia, Ctenomyidae). J Mamm, 72, 11–21.
Gallardo, M H, and Anrioue, J. 1991. Populational parameters and burrow systems in Ctenomys maulinus brunneus (Rodentia, Ctenomyidae). Med Ambiente, 11, 48–53.
Gallardo, M H, and Köhler, N. 1992. Genetic divergence in Ctenomys (Rodentia, Ctenomyidae) from the Andes of Chile. J Mamm, 72, 99–105.
Gallardo, M H, and Köhler, N. 1994. Demographic changes and genetic losses in populations of fossorial rodents (Ctenomys maulinus brunneus) affected by a natural catastrophe. Z Säugetierkunde, 59 (in press).
Gallardo, M H, and Palma, R E. 1992. Intra- and interspecific genetic variability in Ctenomys (Rodentia, Ctenomyidae). Biochem Syst Ecol, 20, 523–534.
Gallardo, M H, Araneda, C, and Köhler, N. 1992. Genetic divergence in Spalacopus cyanus (Rodentia, Octodontidae). Z Säugetierkunde, 57, 231–237.
Haiduk, M W, Baker, R J, Robbins, L W, and Schlitter, D A. 1981. Chromosomal variation in eight species of African megachiropterans. Cytogenet Cell Genet, 29, 221–232.
Hanski, I. 1991. Single species metapopulation dynamics: concepts, models and observations. Biol J Linn Soc, 42, 17–38.
Hanski, I, and Gilpin, M. 1991. Metapopulation dynamics: brief history and conceptual domain. Biol J Linn Soc, 42, 3–16.
Hartl, D L, and Clark, A G. 1989. Principles of Population Genetics. Sinauer Associates, Sunderland, MA.
Harwood, J, and Hall, A. 1990. Mass mortality in marine mammals: its implications for population dynamics and genetics. Trends Ecol Evol, 5, 254–257.
Husband, B C, and Barrett, S C H. 1992. Effective population size and genetic drift in Eichhornia paniculata. Evolution, 46, 1875–1890.
Johnson, M S. 1988. Founder effects and geographic variation in the land snail Theba pisana. Heredity, 61, 133–142.
Lande, R. 1987. Extinction thresholds in demographic models of territorial populations. Am Nat, 130, 624–635.
Lande, R. 1988. Genetics and demography in biological conservation. Science, 241, 1455–1460.
Lande, R, and Barrowclough, G F. 1987. Effective population size, genetic variation and their use in population management. In: Soulé, M. E. (ed.) Viable Populations for Conservation, pp. 87–123. Cambridge University Press, New York.
Leberg, P. 1992. Effects of population bottlenecks on genetic diversity as measured by enzyme electrophoresis. Evolution, 46, 477–494.
McCauley, D E. 1991. Genetic consequences of local extinction and recolonization. Trends Ecol Evol, 6, 5–8.
McCommas, S A, and Bryant, E H. 1990. Loss of electro-phoretic variation in serially bottlenecked populations. Heredity, 64, 315–321.
Maruyama, T, and Fuerst, P A. 1985a. Population bottlenecks and nonequilibrium models in population genetics. III. Genie homozygosity in populations which experience periodic bottlenecks. Genetics, 111, 691–703.
Maruyama, T, and Fuerst, P A. 1985b. Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics, 111, 675–689.
Maruyama, T, and Kimura, M. 1980. Genetic variability and effective population size when local extinction and recolonization of subpopulations are frequent. Proc Natl Acad Sci USA, 77, 6710–6714.
Meffert, L M, and Bryant, H E. 1991. Mating propensity and courtship behavior in serially bottlenecked lines of the housefly. Evolution, 45, 293–306.
Moreno, H, and Gardeweg, M C. 1989. La erupcion reciente en el complejo volcánico Lonquimay (Diciembre 1988). Andes del Sur Rev Geol Chile, 16, 93–117.
Nei, M, Maruyama, T, and Chakraborty, R. 1975. The bottleneck effect and genetic variability in populations. Evolution, 29, 1–10.
Nei, M, and Roychoudhury, A K. 1974. Sampling variances of heterozygosity and genie distance. Genetics, 76, 379–390.
Nei, M, and Tajima, F. 1981. Genetic drift and estimation of effective population size. Genetics, 98, 625–640.
Nevo, E. 1979. Adaptive convergence and divergence of subterranean mammals. Ann Rev Ecol Syst, 10, 269–368.
Nevo, E. 1990. Genetic diversity and its ecological correlates in nature: comparisons between subterranean, fossorial and aboveground small mammals. In: Nevo, E. and Reig, O. A. (eds) Evolution of Subterranean Mammals at the Organismal and Molecular Levels, pp. 347–366. A. R. Liss, New York.
O'Brien, S J, and Everman, J F. 1988. Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol Evol, 3, 254–259.
O'Brien, S J, Roelke, M E, Marker, L, Newman, A, Winkler, C A, Meltzer, D, Colly, L, Vermann, J F, Bush, M, and Wildt, D E. 1985. Genetic basis for species vulnerability in the cheetah. Science, 227, 1428–1435.
O'Brien, S J, Wildt, D E, Bush, M, Caro, T M, Fitzgibbon, C, Aggundey, I, and Leakey, R E. 1987. East African cheetahs: evidence for two population bottlenecks. Proc Natl Acad Sci USA, 84, 508–511.
Packer, C, Pusey, A E, Rowley, H, Gilbert, D A, Martenson, J, and O'Brien, S J. 1991. Case study of a population bottleneck: lions of the Ngorongoro crater. Conserv Biol, 5, 229–237.
Pearsqn, O P. 1959. Biology of subterranean rodents, Ctenomys, in Peru. Mem Mus Hist Nat 'Javier Prado', 9, 1–56.
Pearson, O P, Binstein, N, Boiry, L, Busch, M C, Di Pace, M, Gallopin, G, Penchaszadeh, P, and Piantanida, M. 1968. Estructura social, distribucióon espacialy composicióon poredades de una poblacióon de tuco-tucos (Ctenomys talarum). Invest Zool Chile, 13, 47–80.
Randi, E, and Apollonio, M. 1988. Low biochemical variability in European fallow deer (Dama dama L.): natural bottlenecks and the effects of domestication. Heredity, 61, 405–410.
Reig, O A, Bush, C, Ortells, M O, and Contreras, J R. 1990. An overview of evolution, systematics, population biology, cytogenetics, molecular biology and speciation in Ctenomys. In: Nevo, E. and Reig, O. A. (eds) Evolution of Subterranean Mammals at the Organismal and Molecular Levels, pp. 71–96. A. R. Liss, New York.
Rice, W R, and Hostert, E E. 1993. Laboratory experiments on speciation: what have we learned in 40 years? Evolution, 47, 1637–1653.
Rogers, J S. 1972. Measures of genetic similarity and genetic distance. Studies in genetics VII. Univ Texas Pub I, 7213, 145–153.
Selander, R B, Smith, M H, Yang, S Y, Johnson, W E, and Gentry, J B. 1971. Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the oldfield mouse (Peromyscus polionotus). Studies in genetics VI. Univ Texas Publ, 7103, 49–90.
Simpson, B B. 1979. Quaternary of the high montane regions of South America. In: Duellman, W. R. (ed.) The South American Herpetofauna: its Origin, Evolution and Dispersal Monographs of Museum of University of Kansas, 7, 1–485.
Slatkin, M. 1993. Isolation by distance in equilibrium and non-equilibrium populations. Evolution, 47, 264–279.
Swofford, D L, and Selander, R B. 1989. BIOSYS-1. A computer program for the analysis of allelic variation in population genetics and biochemical systematics Illinois Nat. Hist. Survey, IL.
Veblen, T T. 1985. Stand dynamics in Chilean Nothofagus forests. In: Pickett, S. T. A. and White, P. S. (eds) The Ecology of Natural Disturbance and Patch Dynamics, pp. 35–51. Academic Press, London.
Wade, M J, and McCauley, D E. 1988. Extinction and recolonization: their effects on the genetic differentiation of local populations. Evolution, 42, 995–1005.
Wildt, D E, Bush, M, Goodgrowe, K L, Packer, C, Pusey, A E, Brown, J L, Joslin, P, and O'Brien, S J. 1987. Reproductive and genetic consequences of founding isolated lion populations. Science, 329, 328–330.
Workman, P L, and Niswander, J D. 1970. Population studies on Southwestern Indian tribes. II. Local genie differentiation in the Papago. Am J Hum Genet, 22, 24–49.
Wright, S. 1965. The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution, 19, 395–420.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gallardo, M., Köhler, N. & Araneda, C. Bottleneck effects in local populations of fossorial Ctenomys (Rodentia, Ctenomyidae) affected by vulcanism. Heredity 74, 638–646 (1995). https://doi.org/10.1038/hdy.1995.87
Received:
Issue date:
DOI: https://doi.org/10.1038/hdy.1995.87
Keywords
This article is cited by
-
Changes in selection intensity on the mitogenome of subterranean and fossorial rodents respective to aboveground species
Mammalian Genome (2018)
-
Genomic data reveal a loss of diversity in two species of tuco-tucos (genus Ctenomys) following a volcanic eruption
Scientific Reports (2017)
-
The successful founder: genetics of introduced Carduelis chloris (greenfinch) populations in New Zealand
Heredity (1996)