Abstract
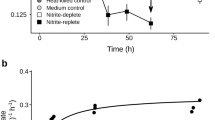
Permafrost-affected soils of the Siberian Arctic were investigated with regard to identification of nitrite oxidizing bacteria active at low temperature. Analysis of the fatty acid profiles of enrichment cultures grown at 4°C, 10°C and 17°C revealed a pattern that was different from that of known nitrite oxidizers but was similar to fatty acid profiles of Betaproteobacteria. Electron microscopy of two enrichment cultures grown at 10°C showed prevalent cells with a conspicuous ultrastructure. Sequence analysis of the 16S rRNA genes allocated the organisms to a so far uncultivated cluster of the Betaproteobacteria, with Gallionella ferruginea as next related taxonomically described organism. The results demonstrate that a novel genus of chemolithoautotrophic nitrite oxidizing bacteria is present in polygonal tundra soils and can be enriched at low temperatures up to 17°C. Cloned sequences with high sequence similarities were previously reported from mesophilic habitats like activated sludge and therefore an involvement of this taxon in nitrite oxidation in nonarctic habitats is suggested. The presented culture will provide an opportunity to correlate nitrification with nonidentified environmental clones in moderate habitats and give insights into mechanisms of cold adaptation. We propose provisional classification of the novel nitrite oxidizing bacterium as ‘Candidatus Nitrotoga arctica’.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Aamand J, Ahl T, Spieck E . (1996). Monoclonal antibodies recognizing nitrite oxidoreductase of Nitrobacter hamburgensis, N. winogradskyi, and N. vulgaris. Appl Environ Microbiol 62: 2352–2355.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ . (1990). Basic local alignment search tool. J Mol Biol 215: 403–410.
Avrahami S, Conrad R . (2003). Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl Environ Microbiol 69: 6152–6164.
Bartosch S, Hartwig C, Spieck E, Bock E . (2002). Immunological detection of Nitrospira-like bacteria in various soils. Microb Ecol 43: 26–33.
Bartosch S, Wolgast I, Spieck E, Bock E . (1999). Identification of nitrite oxidizing bacteria with monoclonal antibodies recognizing the nitrite oxidoreductase. Appl Environ Microbiol 65: 4126–4133.
Brümmer IHM, Felske A, Wagner-Döbler I . (2003). Diversity and seasonal variability of β-Proteobacteria in Biofilms of polluted rivers: analysis by temperature gradient gel electrophoresis and cloning. Appl Environ Microbiol 69: 4463–4473.
Cole JR, Chai B, Farris RJ, Wang Q, Kulam-Syed-Mohideen AS, McGarrell DM et al. (2007). The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res 35 (database issue): D169–D172; doi:10.1093/nar/gkl889.
Ehrich S, Behrens D, Lebedeva E, Ludwig W, Bock E . (1995). A new obligately chemolithoautotrophic, nitrite oxidizing bacterium, Nitrospira moscoviensis sp: nov and its phylogenetic relationship. Arch Microbiol 164: 16–23.
Jones RD, Morita R . (1985). Low-temperature growth and whole cell kinetics of a marine ammonium oxidizer. Mar Ecol Prog Ser 21: 239–243.
Jones RD, Prahl FG . (1985). Lipid composition of a marine ammonium oxidizer grown at 5 and 25°C. Mar Ecol Prog Ser 26: 157–159.
Kobabe S, Wagner D, Pfeiffer E-M . (2004). Characterization of microbial composition of Siberian tundra soil by fluorescence in situ hybridization. FEMS Microbiol Ecol 50: 13–23.
Könneke M, Bernhard AE, De La Torre JR, Walker CB, Waterbury J, Stahl DA . (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546.
Lane DJ . (1991). 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds). Nucleic Acid Techniques in Bacterial Systematics. Academic Press: Chichester, UK, pp 115–175.
Lebedeva EV, Alawi M, Fiencke C, Namsaraev B, Bock E, Spieck E . (2005). Moderately thermophilic nitrifying bacteria from a hot spring of the Baikal rift zone. FEMS Microbiol Ecol 54: 297–306.
Lebedeva E, Soina V . (1994). Nitrifying bacteria as a promising group for paleoecological investigations in the permafrost. In: Gilichinsky DA (ed). Viable Microorganisms in Permafrost. First International Conference on Cryopedology and Global Change. Russian Academy of Sciences Pushino Research Centre, ISBN 5-206-10586-6, pp 74–82.
Liebner S, Wagner D . (2007). Abundance, distribution and potential activity of methane oxidizing bacteria in permafrost soils from the Lena Delta, Siberia. Environ Microbiol 9: 107–117.
Lipski A, Spieck E, Makolla A, Altendorf K . (2001). Fatty acid profiles of nitrite-oxidizing bacteria reflect their phylogenetic heterogeneity. Syst Appl Microbiol 24: 377–384.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhu K et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H . (1992). Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol 15: 593–600.
Meincke M, Bock E, Kastrau D, Kroneck PMH . (1992). Nitrite oxidoreductase from Nitrobacter hamburgensis: redox centers and their catalytic role. Arch Microbiol 158: 127–131.
Murray RG, Stackebrandt E . (1995). Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int J Syst Bacteriol 45: 186–187.
Muyzer G, Brinkhoff T, Nübel U, Santegoeds C, Schäfer H, Wawer C . (1998). Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. Mol Microbial Ecol Manual 3.4.4: 1–27.
Philips S, Laanbroek HJ, Verstraete W . (2002). Origin, causes, and effects of increased nitrite concentrations in aquatic environments. Rev Environ Sci Biotechnol 1: 115–141.
Ponder M, Gilmour SJ, Bergholz PW, Mindock CA, Hollinsworth R, Thomashow et al. (2005). Characterization of potential stress responses in ancient Siberian permafrost psychroactive bacteria. FEMS Microbiol Ecol 53: 103–115.
Schwarz JI, Eckert W, Conrad R . (2007). Community structure of Archaea and Bacteria in a profundal lake sediment Lake Kinneret (Israel). Syst Appl Microbiol 30: 239–254.
Skidmore M, Anderson SP, Sharp M, Foght J, Lanoil BD . (2005). Comparison of microbial community compositions of two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microbiol 71: 6986–6997.
Spieck E, Bock E . (2005). The lithoautotrophic nitrite-oxidizing bacteria. In: Garrity G, Brenner DJ, Krieg NR, Staley JT (eds). Bergey's Manual of Systematic Bacteriology, 2nd edn. Springer-Verlag: New York, NY, pp 149–153.
Spieck E, Hartwig C, McCormack I, Maixner F, Wagner M, Lipski A et al. (2006). Selective enrichment and molecular characterisation of a previously uncultured Nitrospira-like bacterium from activated sludge. Environ Microbiol 8: 405–415.
Steinmüller W, Bock E . (1976). Growth of Nitrobacter in the presence of organic matter; I. Mixotrophic growth. Arch Microbiol 108: 299–304.
Wagner D, Lipski A, Embacher A, Gattinger A . (2005). Methane fluxes in permafrost habitats of the Lena Delta: effects of microbial community structure and organic matter quality. Environ Microbiol 7: 1582–1592.
Wagner D, Spieck E, Pfeiffer E-M, Bock E . (2001). Microbial life in terrestrial permafrost: Methanogenesis and nitrification in gelisols as potentials for exobiological processes. In: Horneck G, Baumstark-Khan C (eds.) Astrobiologie-the Quest for the Conditions of Life. Springer-Verlag: Berlin, NY, pp 143–159.
Watson SW, Bock E, Valois FW, Waterbury JB, Schlosser U . (1986). Nitrospira marina gen. nov. sp. nov: a chemolithotrophic nitrite-oxidizing bacterium. Arch Microbiol 144: 1–7.
Watson SW, Bock E, Harms H, Koops H-P, Hooper AB . (1989). Nitrifying bacteria. In: Staley JT, Bryant MP, Pfennig N, Holt JG (eds). Bergey's Manual of Systematic Bacteriology, 1st edn, Vol. 3. The Williams & Wilkins Co: Baltimore, MD, pp 1808–1834.
Zhou J, Davey ME, Figuera JB, Rivkina E, Gilichinsky D, Tiedje JM . (1997). Phylogenetic diversity of a bacterial community determined from Siberian tundra soil DNA. Microbiology 143: 3913–3919.
Acknowledgements
Sampling was kindly performed by D Wagner (AWI Potsdam) in frame of the German–Russian cooperation ‘System Laptev Sea’. We thank I Wachholz for technical help in electron microscopy, P-G Jozsa for chemical analysis by HPLC-technique and M Kaya for culturing nitrite oxidizers from activated sludge. FISH analysis was kindly supported by C Hartwig. Laboratory assistance was provided by M Klimova (Voronezh State University) in the frame of a DAAD exchange programe. This research was funded by the BMBF Russian–German Cooperation ‘Laptev Sea System: Process Studies on Permafrost Dynamics in the Laptev Sea’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alawi, M., Lipski, A., Sanders, T. et al. Cultivation of a novel cold-adapted nitrite oxidizing betaproteobacterium from the Siberian Arctic. ISME J 1, 256–264 (2007). https://doi.org/10.1038/ismej.2007.34
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2007.34
Keywords
This article is cited by
-
Diversity and distribution of iron-oxidising bacteria belonging to Gallionellaceae in different sites of a hydroelectric power plant
Brazilian Journal of Microbiology (2024)
-
Relevance of Candidatus Nitrotoga for nitrite oxidation in technical nitrogen removal systems
Applied Microbiology and Biotechnology (2021)
-
Shifts in the Abundance and Community Composition of Particle-Associated and Free-Living Nitrospira Across Physicochemical Gradients in the Pearl River Estuary
Estuaries and Coasts (2021)
-
The Nitrogen-Cycling Network of Bacterial Symbionts in the Sponge Spheciospongia vesparium
Journal of Ocean University of China (2021)
-
Spatio-Temporal Variations in the Abundance and Community Structure of Nitrospira in a Tropical Bay
Current Microbiology (2020)