Abstract
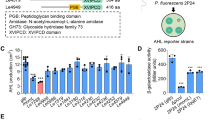
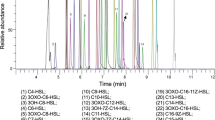
The overall goal of this study was to examine the role of quorum-sensing (QS) signals in a multispecies microbial community. Toward this aim, we studied QS signals produced by an indigenous member and an invading pathogen of the microbial community of the cabbage white butterfly (CWB) larval midgut (Pieris rapae). As an initial step, we characterized the QS system in Pantoea CWB304, which was isolated from the larval midgut. A luxI homolog, designated panI, is necessary for the production of N-acyl-L-homoserine lactones (AHLs) by Pantoea CWB304. To determine whether AHL signals are exchanged in the alkaline environment of the midgut, we constructed AHL-sensing bioluminescent reporter strains in Pantoea CWB304 and a panI mutant of this strain. In the gut of the CWB larvae, the reporter in an AHL-deficient Pantoea CWB304 detected AHLs when coinoculated with the wild type. To study the role of AHL signals produced by a community invader, we examined pathogenesis of Pseudomonas aeruginosa PAO1 in CWB larvae. Mortality induced by P. aeruginosa PAO1 was significantly reduced when signaling was interrupted by either a potent chemical inhibitor of QS or mutations in the lasI and rhlI AHL synthases of P. aeruginosa PAO1. These results show that AHLs are exchanged among bacteria in the alkaline gut of CWB larvae and contribute to disease caused by P. aeruginosa PAO1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402.
Andersen JB, Heydorn A, Hentzer M, Eberl L, Geisenberger O, Christensen BB et al. (2001). gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl Environ Microbiol 67: 575–585.
Berenbaum M (1980). Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115: 138–146.
Broderick NA, Raffa KF, Goodman RM, Handelsman J . (2004). Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol 70: 293–300.
Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y et al. (2003). The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl Environ Microbiol 69: 4989–4993.
Cui Y, Chatterjee A, Liu Y, Dumenyo CK, Chatterjee AK . (1995). Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-L-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J Bacteriol 177: 5108–5115.
Dong YH, Gusti AR, Zhang Q, Xu JL, Zhang LH . (2002). Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl Environ Microbiol 68: 1754–1759.
Engebrecht J, Silverman M . (1984). Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA 81: 4154–4158.
Fuqua WC, Winans SC, Greenberg EP . (1994). Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176: 269–275.
Geske GD, Wezeman RJ, Siegel AP, Blackwell HE . (2005). Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J Am Chem Soc 127: 12762–12763.
Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L et al. (1996). Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol 178: 6618–6622.
Guan C, Ju J, Borlee BR, Williamson LL, Shen B, Raffa KF et al. (2007). Signal mimics derived from a metagenomic analysis of the gypsy moth gut microbiota. Appl Environ Microbiol 73: 3669–3676.
Latifi A, Winson MK, Foglino M, Bycroft BW, Stewart GS, Lazdunski A et al. (1995). Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol 17: 333–343.
Leadbetter JR, Greenberg EP . (2000). Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol 182: 6921–6926.
Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP . (2006). A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J Bacteriol 188: 3365–3370.
Lesprit P, Faurisson F, Join-Lambert O, Roudot-Thoraval F, Foglino M, Vissuzaine C et al. (2003). Role of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in rats. Am J Respir Crit Care Med 167: 1478–1482.
Lin YH, Xu JL, Hu J, Wang LH, Ong SL, Leadbetter JR et al. (2003). Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol Microbiol 47: 849–860.
Lithgow JK, Wilkinson A, Hardman A, Rodelas B, Wisniewski-Dye F, Williams P et al. (2000). The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol Microbiol 37: 81–97.
Manefield M, Turner SL . (2002). Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology 148: 3762–3764.
Martinelli D, Grossmann G, Sequin U, Brandl H, Bachofen R . (2004). Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol 4: 25.
McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M et al. (1997). Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143: 3703–3711.
McGrath S, Wade DS, Pesci EC . (2004). Dueling quorum-sensing systems in Pseudomonas aeruginosa control the production of the Pseudomonas quinolone signal (PQS). FEMS Microbiol Lett 230: 27–34.
Michael B, Smith JN, Swift S, Heffron F, Ahmer BM . (2001). SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J Bacteriol 183: 5733–5742.
Miller MB, Bassler BL . (2001). Quorum sensing in bacteria. Annu Rev Microbiol 55: 165–199.
Park SY, Kang HO, Jang HS, Lee JK, Koo BT, Yum DY . (2005). Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl Environ Microbiol 71: 2632–2641.
Park SY, Lee SJ, Oh TK, Oh JW, Koo BT, Yum DY et al. (2003). AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology 149: 1541–1550.
Pearson JP, Feldman M, Iglewski BH, Prince A . (2000). Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun 68: 4331–4334.
Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, Iglewski BH et al. (1994). Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91: 197–201.
Pearson JP, Passador L, Iglewski BH, Greenberg EP . (1995). A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92: 1490–1494.
Pearson JP, Pesci EC, Iglewski BH . (1997). Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179: 5756–5767.
Piper KR, Beck von Bodman S, Farrand SK . (1993). Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362: 448–450.
Riedel K, Hentzer M, Geisenberger O, Huber B, Steidle A, Wu H et al. (2001). N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147: 3249–3262.
Steidle A, Sigl K, Schuhegger R, Ihring A, Schmid M, Gantner S et al. (2001). Visualization of N-acylhomoserine lactone-mediated cell–cell communication between bacteria colonizing the tomato rhizosphere. Appl Environ Microbiol 67: 5761–5770.
Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM . (1999). Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA 96: 2408–2413.
Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG . (2000). Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol 182: 4578–4586.
von Bodman SB, Majerczak DR, Coplin DL . (1998). A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc Natl Acad Sci USA 95: 7687–7692.
Williamson LL, Borlee BR, Schloss PD, Guan C, Allen HK, Handelsman J . (2005). Intracellular screen to identify metagenomic clones that induce or inhibit a quorum-sensing biosensor. Appl Environ Microbiol 71: 6335–6344.
Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR et al. (1998). Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163: 185–192.
Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T et al. (2004). Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci USA 101: 4859–4864.
Yates EA, Philipp B, Buckley C, Atkinson S, Chhabra SR, Sockett RE et al. (2002). N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect Immun 70: 5635–5646.
Acknowledgements
We are grateful to Ronald Binder for generously providing synthetic AHLs; to Professors Leo Eberl, Michael Givskov, Max Teplitski and Barbara Iglewski for providing plasmids and strains; and to Gary Splitter and Gireesh Rajashekara for the assistance with and use of the IVIS BLI 100 CCD camera. We are also grateful to Professor Kenneth Raffa for guidance and use of his insect-rearing facilities and Anders Boyd for sharing data on the midgut pH. BRB was supported by an Advanced Opportunity Fellowship from the University of Wisconsin–Madison Graduate School. This work was supported by the Howard Hughes Medical Institute, NIH Grant no. 5RO1GM75830-2 and the University of Wisconsin–Madison College of Agricultural and Life Sciences Hatch Project no. 4038. Research in the Blackwell lab is supported in part by the NSF (CHE-0449959), Burroughs Welcome Foundation, Johnson & Johnson and Greater Milwaukee Foundation Shaw Scientist Program. HEB is an Alfred P Sloan Foundation Fellow and a Research Corporation Cottrell Scholar. GDG was supported by an American Chemical Society Division of Medicinal Chemistry predoctoral fellowship. CJR was supported by the Biotechnology Training Program (NIH 5 T32 GM08349) and an Advanced Opportunity Fellowship from the University of Wisconsin–Madison Graduate School.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borlee, B., Geske, G., Robinson, C. et al. Quorum-sensing signals in the microbial community of the cabbage white butterfly larval midgut. ISME J 2, 1101–1111 (2008). https://doi.org/10.1038/ismej.2008.70
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2008.70
Keywords
This article is cited by
-
N-acyl-homoserine lactone produced by Rahnella inusitata isolated from the gut of Galleria mellonella influences Salmonella phenotypes
Brazilian Journal of Microbiology (2022)
-
Multifaceted interactions between the pseudomonads and insects: mechanisms and prospects
Archives of Microbiology (2021)
-
Sex-specific asymmetry within the cloacal microbiota of the striped plateau lizard, Sceloporus virgatus
Symbiosis (2010)
-
Robustness of the Bacterial Community in the Cabbage White Butterfly Larval Midgut
Microbial Ecology (2010)