Abstract
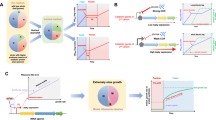
The natural habitats of microbes are typically spatially structured with limited resources, so opportunities for unconstrained, balanced growth are rare. In these habitats, selection should favor microbes that are able to use resources most efficiently, that is, microbes that produce the most progeny per unit of resource consumed. On the basis of this assertion, we propose that selection for efficiency is a primary driver of the composition of microbial communities. In this article, we review how the quality and quantity of resources influence the efficiency of heterotrophic growth. A conceptual model proposing innate differences in growth efficiency between oligotrophic and copiotrophic microbes is also provided. We conclude that elucidation of the mechanisms underlying efficient growth will enhance our understanding of the selective pressures shaping microbes and will improve our capacity to manage microbial communities effectively.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bachmann H, Fischlechner M, Rabbers I, Barfa N, Branco Dos Santos F, Molenaar D et al. (2013). Availability of public goods shapes the evolution of competing metabolic strategies. Proc Natl Acad Sci USA 110: 14302–14307.
Cavicchioli R, Ostrowski M, Fegatella F, Goodchild A, Guixa-Boixereu N . (2003). Life under nutrient limitation in oligotrophic marine environments: an eco/physiological perspective of Sphingopyxis alaskensis (formerly Sphingomonas alaskensis). Microbl Ecol 45: 203–217.
Cho BC, Azam F . (1988). Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 332: 441–443.
Chuang J, Rivoire O, Leibler S . (2009). Simpson's paradox in a synthetic microbial system. Science 323: 272–275.
Cordier J, Butsch B, Birou B, Stockar U . (1987). The relationship between elemental composition and heat of combustion of microbial biomass. Appl Microbiol Biotechnol 25: 305–312.
Dykhuizen D, Hartl D . (1981). Evolution of competitive ability in Escherichia coli. Evolution, 581–594.
Fierer N, Bradford M, Jackson R . (2007). Toward an ecological classification of soil bacteria. Ecology 88: 1354–1364.
Flamholz A, Noor E, Bar-Even A, Liebermeister W, Milo R . (2013). Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc Natl Acad Sci USA 110: 10039–10044.
Giovannoni SJ, Thrash JC, Temperton Ben . (2014). Implications of streamlining theory for microbial ecology. ISME J 8: 1553–1565.
Herbert D . (1976). Stoicheiometric aspects of microbial growth. In Dean A, Ellwood DC, Evans C, Melling J, (eds) Continuous Culture 6: Applications and New Fields. Ellis Horwood: Chichester, UK, pp 1–30.
Hoehler TM, Jørgensen BB . (2013). Microbial life under extreme energy limitation. Nat Rev Microbiol 11: 83–94.
Koch A . (2001). Oligotrophs versus copiotrophs. Bioessays 23: 657–661.
Lauro F, McDougald D, Thomas T, Williams T, Egan S, Rice S et al. (2009). The genomic basis of trophic strategy in marine bacteria. Proc Natil Acad Sci USA 106: 15527–15533.
Lee ZM, Schmidt TM . (2014). Bacterial growth efficiency varies in soils under different land management practices. Soil Biol Biochem 69: 282–290.
Linton J, Stephenson R . (1978). A preliminary study on growth yields in relation to the carbon and energy content of various organic growth substrates. FEMS Microbiol Lett 3: 95–98.
Maclean RC, Gudelj I . (2006). Resource competition and social conflict in experimental populations of yeast. Nature 441: 498–501.
Manzoni SS, Taylor PP, Richter AA, Porporato AA, Agren GIG . (2012). Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196: 79–91.
McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA 110: 3229–3236.
Morris JJ, Lenski RE, Zinser ER . (2012). The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio 3: e00036–12.
Neidhardt FC . (1999). Bacterial growth: constant obsession with dN/dt. J Bacteriol 181: 7405–7408.
Neijssel O, Tempest D . (1976). Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol 107: 215–221.
Neijssel O, Tempest D . (1975). The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms, growing in chemostat culture. Arch Microbiol 106: 251–258.
Owens J, Legan J . (1987). Determination of the Monod substrate saturation constant for microbial growth. FEMS Microbiol Lett 46: 419–432.
Pfeiffer T, Schuster S, Bonhoeffer S . (2001). Cooperation and competition in the evolution of ATP-producing pathways. Science 292: 504–507.
Russell JB, Cook GM . (1995). Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev 59: 48–62.
Schaechter M, Maaloe O, Kjeldgaard N . (1958). Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. Microbiology 19: 592.
Simon M, Azam F . (1989). Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51: 201–213.
Singh BK, Bardgett RD, Smith P, Reay DS . (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8: 779–790.
Stocker R . (2012). Marine microbes see a sea of gradients. Science 338: 628–633.
Stouthamer AH . (1973). A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek 39: 545–565.
Tempest D, Neijssel O . (1984). The status of YATP and maintenance energy as biologically interpretable phenomena. Ann Rev Microbiol 38: 459–513.
Trivedi P, Anderson IC, Singh BK . (2013). Microbial modulators of soil carbon storage: integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 21: 641–651.
van Bodegom P . (2007). Microbial maintenance: a critical review on its quantification. Microb Ecol 53: 513–523.
Vasi F, Travisano M, Lenski RE . (1994). Long-term experimental evolution in Escherichia coli. II. Changes in life-history traits during adaptation to a seasonal environment. Am Nat, 432–456.
Vergin KL, Done B, Carlson CA, Giovannoni SJ . (2013). Spatiotemporal distributions of rare bacterioplankton populations indicate adaptive strategies in the oligotrophic ocean. Aquat Microb Ecol 71: 1–13.
Zhao Y, Temperton Ben, Thrash JC, Schwalbach MS, Vergin KL, Landry ZC et al. (2013). Abundant SAR11 viruses in the ocean. Nature 494: 357–360.
Acknowledgements
We would like to acknowledge Arvind Venkataraman, Byron Smith, Clive Waldron, Alex Schmidt and Zarraz Lee for valuable feedback throughout the writing process. This work was supported in part by the Department of Energy Office of Science Graduate Fellowship Program (DOE SCGF), made possible in part by the American Recovery and Reinvestment Act of 2009, administered by ORISE-ORAU under contract no. DE-AC05-06OR23100; the National Science Foundation’s Long-Term Ecological Research Program through grant no. DEB 1027253 and the National Institutes of Health (GM0099549).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Roller, B., Schmidt, T. The physiology and ecological implications of efficient growth. ISME J 9, 1481–1487 (2015). https://doi.org/10.1038/ismej.2014.235
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2014.235
This article is cited by
-
Relationships between intrinsic population growth rate, carrying capacity and metabolism in microbial populations
The ISME Journal (2023)
-
Double emulsions as a high-throughput enrichment and isolation platform for slower-growing microbes
ISME Communications (2023)
-
Low-temperature corn straw-degrading bacterial agent and moisture effects on indigenous microbes
Applied Microbiology and Biotechnology (2023)
-
Translation Comes First: Ancient and Convergent Selection of Codon Usage Bias Across Prokaryotic Genomes
Journal of Molecular Evolution (2022)
-
Land use driven change in soil pH affects microbial carbon cycling processes
Nature Communications (2018)