Abstract
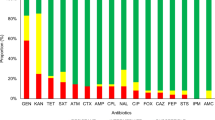
Escherichia coli are one of the leading causes of infection in wounds. Emerging multiple drug resistance among E. coli poses a serious challenge to antimicrobial therapy for wounds. This study was conducted to ascertain a baseline profile of antimicrobial resistance in E. coli isolates infecting surgical wounds. A total of 64 pus samples from hospitalized patients were screened and 29 (45.3%) were found to have E. coli, which were identified biochemically and confirmed by molecular methods. Using the disc diffusion method, antimicrobial resistance was observed toward tetracycline (100%), cefradine (100%), nalidixic acid (93.1%), ampicillin (86.2%), gentamicin (86.2%), cefixime (82.8%), ceftriaxone (82.8%), aztreonam (82.8%), ciprofloxacin (75.9%), streptomycin (72.4%), cefoperazone (65.5%), chloramphenicol (58.6%) and amikacin (58.6%). In an effort to find relevant genes, 11 different genes were targeted by PCR. Among these, the mutated gyrA gene was found to be the most prevalent (82.8%), followed by the TEM (72.4%), catP (68.9%), catA1 (68.9%), tetB (62.1%), blt (58.6%), blaCTX−M−15 (27.6%), blaTEM (20.7%), blaOXA (17.2%), tetA (17.2%) and aadA1 (13.8%) genes. The presence of integrons was also studied among these isolates. The prevalence of class 1 integrons was the highest (44.8%), followed by class 2 (27.6%). Three (10.3%) isolates carried both class 1 and class 2 integrons (first report from E. coli infecting wounds). The high incidence of integrons points toward their facilitation for carriage of antimicrobial resistance genes; however, in nearly 37% isolates, no integrons were detected, indicating the significance of alternative mechanisms of gene transfer. Another salient finding was that all isolates were multidrug-resistant E. coli.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Russo, T. A. & Johnson, J. R. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes. Infect. 5, 449–456 (2003).
Anguzu, J. R. & Olila, D. Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. Afr. Health. Sci. 7, 148–154 (2007).
Rowe-Magnus, D. A. & Mazel, D. The role of integrons in antibiotic resistance gene capture. Int. J. Med. Microbiol. 292, 115–125 (2002).
Srinivasan, V. et al. Characterization of antimicrobial resistance patterns and class 1 integrons in Escherichia coli O26 isolated from humans and animals. Int. J. Antimicrob. Agents. 29, 254–262 (2007).
Zhao, S. et al. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet. Microbiol. 107, 215–224 (2005).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular cloning: A laboratory manual (Cold Spring Harbor Laboratory Press, New York, 1989).
National Committe for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Fourth informational supplement. Document M100-S14 (NCCLS: Wayne, PA, 2004).
Shanahan, P. M., Karamat, K. A., Thomson, C. J. & Amyes, S. G. Characterization of multi-drug resistant Salmonella typhi isolated from Pakistan. Epidemiol. Infect. 124, 9–16 (2000).
Hawkey, P. M. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin. Microbiol. Infect. 14 (Suppl 1), 159–165 (2008).
Reyes, A. et al. Prevalence and types of class 1 integrons in aminoglycoside-resistant Enterobacteriaceae from several Chilean hospitals. J. Antimicrob. Chemother. 51, 317–321 (2003).
Yu, H. S. et al. Changes in gene cassettes of class 1 integrons among Escherichia coli isolates from urine specimens collected in Korea during the last two decades. J. Clin. Microbiol. 41, 5429–5433 (2003).
Mathai, E., Grape, M. & Kronvall, G. Integrons and multidrug resistance among Escherichia coli causing community-acquired urinary tract infection in southern India. APMIS. 112, 159–164 (2004).
Solberg, O. D., Ajiboye, R. M. & Riley, L. W. Origin of class 1 and 2 integrons and gene cassettes in a population-based sample of uropathogenic Escherichia coli. J. Clin. Microbiol. 44, 1347–1351 (2006).
Skurnik, D. et al. Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob. Agents. Chemother. 49, 3062–3065 (2005).
Nagoba, B. S. et al. A simple and effective approach for the treatment of chronic wound infections caused by multiple antibiotic resistant Escherichia coli. J. Hosp. Infect. 69, 177–180 (2008).
Piatti, G., Mannini, A., Balistreri, M. & Schito, A. M. Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J. Clin. Microbiol. 46, 480–487 (2008).
Mehrgan, H. & Rahbar, M. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int. J. Antimicrob. Agents. 31, 147–151 (2008).
Van, T. T., Chin, J., Chapman, T., Tran, L. T. & Coloe, P. J. Safety of raw meat and shellfish in Vietnam: an analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food. Microbiol. 124, 217–223 (2008).
Jabeen, K., Zafar, A. & Hasan, R. Frequency and sensitivity pattern of extended spectrum beta lactamase producing isolates in a tertiary care hospital laboratory of Pakistan. J. Pak. Med. Assoc. 55, 436–439 (2005).
Guardabassi, L., Dijkshoorn, L., Collard, J. M., Olsen, J. E. & Dalsgaard, A. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J. Med. Microbiol. 49, 929–936 (2000).
Srinivasan, V., Nguyen, L. T., Headrick, S. I., Murinda, S. E. & Oliver, S. P. Antimicrobial resistance patterns of Shiga toxin-producing Escherichia coli O157:H7 and O157:H7- from different origins. Microb. Drug. Resist. 13, 44–51 (2007).
Enne, V. I., Delsol, A. A., Roe, J. M. & Bennett, P. M. Evidence of antibiotic resistance gene silencing in Escherichia coli. Antimicrob. Agents. Chemother. 50, 3003–3010 (2006).
Heininger, A. et al. PCR and blood culture for detection of Escherichia coli bacteremia in rats. J. Clin. Microbiol. 37, 2479–2482 (1999).
Carlson, S. A. et al. Detection of multiresistant Salmonella typhimurium DT104 using multiplex and fluorogenic PCR. Mol. Cell. Probes. 13, 213–222 (1999).
Chu, C. et al. Large drug resistance virulence plasmids of clinical isolates of Salmonella enterica serovar Choleraesuis. Antimicrob. Agents. Chemother. 45, 2299–2303 (2001).
Peirano, G., Agerso, Y., Aarestrup, F. M. & dos Prazeres Rodrigues, D. Occurrence of integrons and resistance genes among sulphonamide-resistant Shigella spp. from Brazil. J. Antimicrob. Chemother. 55, 301–305 (2005).
Molbak, K. et al. An outbreak of multidrug-resistant, quinolone-resistant Salmonella enterica serotype typhimurium DT104. N. Engl. J. Med. 341, 1420–1425 (1999).
Guerra, B., Soto, S. M., Arguelles, J. M. & Mendoza, M. C. Multidrug resistance is mediated by large plasmids carrying a class 1 integron in the emergent Salmonella enterica serotype [4,5,12:i:-]. Antimicrob. Agents. Chemother. 45, 1305–1308 (2001).
Yan, J. J. et al. Dissemination of CTX-M-3 and CMY-2 beta-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J. Clin. Microbiol. 38, 4320–4325 (2000).
Mendonca, N., Leitao, J., Manageiro, V., Ferreira, E. & Canica, M. Spread of extended-spectrum beta-lactamase CTX-M-producing Escherichia coli clinical isolates in community and nosocomial environments in Portugal. Antimicrob. Agents. Chemother. 51, 1946–1955 (2007).
Rosser, S. J. & Young, H. K. Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J. Antimicrob. Chemother. 44, 11–18 (1999).
Senda, K. et al. PCR detection of metallo-beta-lactamase gene (blaIMP) in Gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin Microbiol. 34, 2909–2913 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeed, M., Haque, A., Ali, A. et al. A profile of drug resistance genes and integrons in E. coli causing surgical wound infections in the Faisalabad region of Pakistan. J Antibiot 62, 319–323 (2009). https://doi.org/10.1038/ja.2009.37
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2009.37
Keywords
This article is cited by
-
Punicalagin, a pomegranate polyphenol sensitizes the activity of antibiotics against three MDR pathogens of the Enterobacteriaceae
BMC Complementary Medicine and Therapies (2024)
-
Antibiotic resistance in Pakistan: a systematic review of past decade
BMC Infectious Diseases (2021)
-
Antimicrobial resistance among GLASS priority pathogens from Pakistan: 2006–2018
BMC Infectious Diseases (2021)
-
Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria
BMC Complementary and Alternative Medicine (2013)