Abstract
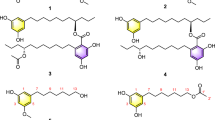
Four new alkaloids, including two new meleagrin analogs, meleagrin D (1) and E (2), and two new diketopiperazines, roquefortine H (3) and I (4), were isolated from a deep ocean sediment-derived fungus Penicillium sp. Meleagrin D (1) and E (2) possess unprecedented acetate–mevalonate-derived side chains on the imidazole moiety. These new meleagrins showed weak cytotoxicity against the A-549 cell line, whereas meleagrin B (5) and meleagrin (6), which were isolated previously from the same strain, induced HL-60 cell apoptosis or arrested the cell cycle through G2/M phase, respectively. The results indicate that the distinct substitutions on the imidazole ring significantly influence the cytotoxicity of the meleagrin alkaloids.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Scott, P. M., Merrien, M. A. & Polonsky, J. Roquefortine and iso-fumigaclavine A, metabolites from Penicillium roqueforti. Experientia. 32, 140–142 (1976).
Ohmomo, S., Oguma, K., Ohashi, T. & Abe, M. Isolation of a new indole alkaloid, roquefortine D, from the cultures of Penicillium roqueforti. Agric. Biol. Chem. 42, 2387–2389 (1978).
Musuku, A et al. Isolation and structure determination of a new roquefortine-related mycotoxin from Penicillium verrucosum VAR. Cyclopium isolated from Cassava. J. Nat. Prod. 57, 983–987 (1994).
Kozlovsky, A. G., Vinokurova, N. G., Solov’eva, T. F. & Buzilova, I. G. Nitrogen-containing secondary metabolites of microscopic fungi. Appl. Biochem. Microbiol. 32, 39–48 (1996).
Kozlovsky, A. G. et al. A new 16n-carboxyethyl derivative of 3,12-dihydroroquefortin. Heterocycles 60, 1639–1644 (2003).
Steyn, P. S. & Vleggaa, R. Roquefortine, an intermediate in the biosynthesis of oxaline in cultures of Penicillium oxalicum. J. Chem. Soc. Chem. Commun. 560–561 (1983).
Clark, B., Capon, R. J., Lacey, E., Tennant, S. & Gill, J. H. Roquefortine E, a diketopiperazine from an Australian isolate of Gymnoascusreessii. J. Nat. Prod. 68, 1661–1664 (2005).
Kopp, B. & Rehm, H. J. Antimicrobial action of roquefortine. Eur. J. Appl. Microbiol. Biotechnol. 6, 397–401 (1979).
Aninat, C., Hayashi, Y., Andre, F. & Delaforge, M. Molecular requirements for inhibition of cytochrome P450 activities by roquefortine. Chem. Res. Toxicol. 14, 1259–1265 (2001).
Koizumi, Y., Arai, M., Tomoda, H. & Omura, S. Oxaline, a fungal alkaloid, arrests the cell cycle in M phaseby inhibition of tubulin polymerization. Biochim. Biophys. Acta 1693, 47–55 (2004).
Du, L. et al. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 65, 1033–1039 (2009).
Kawai, K., Nozawa, K., Nakajima, S. & Iitaka, Y. Studies on fungal products. VII. The structures of meleagrin and 9-O-p-bromobenzoylmeleagrin. Chem. Pharm. Bull. 32, 94–98 (1984).
Yamaguchi, T., Nozawa, K., Nakajima, S., Kawai, K. & Udagawa, S. Absolute configuration of roquefortine C, a tremorgenic mycotoxin. Maikotokishin 34, 29–32 (1991).
Kusch, J. & Rehm, H. J. Regulation aspects of roquefortine production by free and Ca-alginate immobilized mycelia of Penicillium roqueforti. Appl. Microbiol. Biotechnol. 23, 394–399 (1986).
Reshetilova, T. A., Vinokurova, N. G., Khmelenina, V. N. & Kozlovskii, A. G. The role of roquefortine in the synthesis of alkaloids meleagrine, glandicolines-a and glandicolines-b, and oxaline in fungi Penicillium glandicola and P.atramentosum. Microbiology 64, 27–29 (1995).
Williams, R. M., Stoching, E. M. & Sanz-Cervera, J. F. Biosynthesis of prenylated alkaloids derived from tryptophan. Top. Curr. Chem. 209, 97–173 (2000).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
Skehan, P et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl Cancer Inst. 82, 1107–1112 (1990).
Du, L et al. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem. Pharm. Bull. 57, 220–223 (2009).
Acknowledgements
This work was funded by the Chinese Ocean Mineral Resource R & D Association (DY105-2-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, L., Feng, T., Zhao, B. et al. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J Antibiot 63, 165–170 (2010). https://doi.org/10.1038/ja.2010.11
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2010.11
Keywords
This article is cited by
-
Marine Natural Products — a Vital Source of Novel Biotherapeutics
Current Pharmacology Reports (2022)
-
Chemical diversity and biological function of indolediketopiperazines from marine-derived fungi
Marine Life Science & Technology (2020)
-
Isolation and Antibiotic Screening of Fungi from a Hydrothermal Vent Site and Characterization of Secondary Metabolites from a Penicillium Isolate
Marine Biotechnology (2017)
-
Inhibition of biofilm in Bacillus amyloliquefaciens Q-426 by diketopiperazines
World Journal of Microbiology and Biotechnology (2016)
-
Isolation and characterization of bioactive fungi from shark Carcharodon carcharias’ gill with biopharmaceutical prospects
Chinese Journal of Oceanology and Limnology (2016)