Abstract
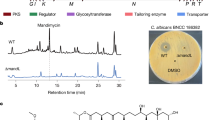
Macrocyclic trichothecenes (MTs), which have potent cytotoxicity, have been isolated from many different fungal species. These compounds were evaluated clinically by the US National Cancer Institute in the 1970s and 1980s. However, they have yet to be advanced into viable drugs because of severe side effects. Our team is investigating a diverse library of filamentous fungi for new anticancer leads. To avoid reisolating MTs through bioactivity-directed fractionation studies, a protocol for their facile dereplication was developed. The method uses readily available photodiode array detectors to identify one of two types of characteristic UV spectra for these compounds. In addition, diagnostic signals can be observed in the 1H-NMR spectra, particularly for the epoxide and conjugated diene moieties, even at the level of a crude extract. Using these techniques in a complementary manner, MTs can be dereplicated rapidly.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cole, R. J., Jarvis, B. B. & Schweikert, M. A. Handbook of Secondary Fungal Metabolites xi, 672 pps (Academic Press, Amsterdam, Boston, 2003).
Zhang, H. J. et al. Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med. 68, 1088–1091 (2002).
Isaka, M., Punya, J., Lertwerawat, Y., Tanticharoen, M. & Thebtaranonth, Y. Antimalarial activity of macrocyclic trichothecenes isolated from the fungus Myrothecium verrucaria. J. Nat. Prod. 62, 329–331 (1999).
Jarvis, B. B. & Mazzola, E. P. Macrocyclic and other novel trichothecenes—their structure, synthesis, and biological significance. Acc. Chem. Res. 15, 388–395 (1982).
Matsumoto, M., Minato, H., Uotani, N., Matsumoto, K. & Kondo, E. New antibiotics from Cylindrocarpon sp. J. Antibiot. 30, 681–682 (1977).
Liu, J. Y. et al. Antifungal and new metabolites of Myrothecium sp. Z16, a fungus associated with white croaker Argyrosomus argentatus. J. Appl. Microbiol. 100, 195–202 (2006).
Garcia, C. C., Rosso, M. L., Bertoni, M. D., Maier, M. S. & Damonte, E. B. Evaluation of the antiviral activity against Junin virus of macrocyclic trichothecenes produced by the hypocrealean epibiont of Baccharis coridifolia. Planta Med. 68, 209–212 (2002).
Bloem, R. J., Smitka, T. A., Bunge, R. H., French, J. C. & Mazzola, E. P. Roridin-L-2, a new trichothecene. Tetrahedron Lett. 24, 249–252 (1983).
Smitka, T. A., Bunge, R. H., Bloem, R. J. & French, J. C. Two new trichothecenes, PD 113,325 and PD 113,326. J. Antibiot. 37, 823–828 (1984).
Wagenaar, M. M. & Clardy, J. Two new roridins isolated from Myrothecium sp. J. Antibiot. 54, 517–520 (2001).
Yu, N. J., Guo, S. X. & Lu, H. Y. Cytotoxic macrocyclic trichothecenes from the mycelia of Calcarisporium arbuscula Preuss. J. Asian Nat. Prod. Res. 4, 179–183 (2002).
Grove, J. F. Macrocyclic trichothecenes. Nat. Prod. Rep. 10, 429–448 (1993).
Hinkley, S. F. & Jarvis, B. B. Chromatographic method for Stachybotrys toxins. Methods Mol. Biol. 157, 173–194 (2001).
Jarvis, B. B., Midiwo, J. O. & Mazzola, E. P. Antileukemic compounds derived by chemical modification of macrocyclic trichothecenes. 2. Derivatives of roridin-A and roridin-H and verrucarin-A and verrucarin-J. J. Med. Chem. 27, 239–244 (1984).
Jarvis, B. B., Stahly, G. P., Pavanasasivam, G. & Mazzola, E. P. Anti-leukemic compounds derived from the chemical modification of macrocyclic trichothecenes. 1. Derivatives of verrucarin-A. J. Med. Chem. 23, 1054–1058 (1980).
Kinghorn, A. D. et al. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 81, 1051–1063 (2009).
Orjala, J., Oberlies, N. H., Pearce, C. J., Swanson, S. M. & Kinghorn, A. D. Discovery of potential anticancer agents from aquatic cyanobacteria, filamentous fungi, and tropical plants. in Bioactive Compounds from Natural Sources 2nd edn, (ed Tringali, C.) (London, Taylor & Francis: submitted).
Nielsen, K. F. & Smedsgaard, J. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 1002, 111–136 (2003).
Lang, G. et al. Evolving trends in the dereplication of natural product extracts: new methodology for rapid, small-scale investigation of natural product extracts. J. Nat. Prod. 71, 1595–1599 (2008).
Grove, J. F. Non-macrocyclic trichothecenes. Nat. Prod. Rep. 5, 187–209 (1988).
Namikoshi, M. et al. A new macrocyclic trichothecene, 12,13-deoxyroridin E, produced by the marine-derived fungus Myrothecium roridum collected in Palau. J. Nat. Prod. 64, 396–398 (2001).
Saikawa, Y. et al. Toxic principles of a poisonous mushroom Podostroma cornu-damae. Tetrahedron 57, 8277–8281 (2001).
Gutzwiller, J. & Tamm, C. Uber die struktur von verrucarin B. Verrucarine und roridine, 6. Helv. Chim. Acta. 48, 177–182 (1965).
Alali, F. Q. et al. New colchicinoids from a native Jordanian meadow saffron, Colchicum brachyphyllum: isolation of the first naturally occurring dextrorotary colchicinoid. J. Nat. Prod. 68, 173–178 (2005).
Li, C. et al. Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod. 72, 1949–1953 (2009).
Acknowledgements
This research was supported by P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. The Golden LEAF Foundation (Rocky Mount, NC, USA) provided partial support to DJK. Mycology technical support was provided by Blaise Darveaux and Maurica Lawrence. We thank Drs AD Kinghorn and DS Ayers for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sy-Cordero, A., Graf, T., Wani, M. et al. Dereplication of macrocyclic trichothecenes from extracts of filamentous fungi through UV and NMR profiles. J Antibiot 63, 539–544 (2010). https://doi.org/10.1038/ja.2010.77
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2010.77
Keywords
This article is cited by
-
Enhanced dereplication of fungal cultures via use of mass defect filtering
The Journal of Antibiotics (2017)
-
Dereplication strategies in natural product research: How many tools and methodologies behind the same concept?
Phytochemistry Reviews (2017)