Abstract
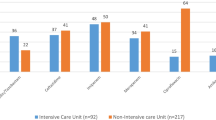
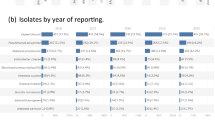
The aim of this study was to determine the antimicrobial resistance rates and the resistance genes associated with efflux pumps of Pseudomonas aeruginosa strains isolated from the patients who acquired lower respiratory tract infection (LRTI) in intensive care unit (ICU). Fifty P. aeruginosa strains isolated from the lower respiratory tract specimens of the patients who acquired LRTIs in ICU were included in this study. P. aeruginosa strains were isolated from tracheal aspirate (27), bronchoalveolar lavage (14) and sputum (9). The susceptibilities of the isolates were investigated by the disk diffusion method. Multiplex PCR assay was carried out for the detection of 13 antibiotic-resistance genes. Antimicrobial resistance rates of the isolates were found high and the highest resistance rate of the isolates studied was determined against to mezlocillin (50%) followed by norfloxacin (48%), ciprofloxacin (46%), meropenem (40%). Fourty-three isolates (86%) were determined to carry one and more resistance genes. NfxB gene was most often determined in the genes that were investigated. The significant relation between the resistance to cefepime, piperacilline/tazobactam and the mexC gene, that between the resistance to mezlocillin, piperacilline/tazobactam, ceftazidime, cefepime and ampC genes, and that between the resistance to ciprofloxacin, norfloxacin and oprJ, oprN and nfxB genes was identified. Resistance caused by genes for carbapenemases, aminoglycoside-modifying enzymes and other mechanisms were not identified in this study. Understanding the prevalence and mechanism of antimicrobial resistance in P. aeruginosa may help to select empirical therapy for nosocomial LRTIs due to P. aeruginosa in our ICU.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hernández, G., Rico, P., Díaz, E. & Rello, J. Nosocomial lung infections in adult intensive care units. Microbes Infect. 6, 1004–1014 (2004).
Vanhems, P. et al. Nosocomial pulmonary infection by antimicrobial-resistant bacteria of patients hospitalized in intensive care units: risk factors and survival. J. Hosp. Infect. 45, 98–106 (2000).
Poole, K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44, 2233–2241 (2000).
Poole, K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3, 255–264 (2001).
Kiska, D. L. & Gilligan, P. H. In: Man. Clin. Microbio. 8th edn (eds Muray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH) (ASM Press, Washington, 2003).
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing-Nineteenth Informational Supplement. CLSI document M100-S21 (Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA, 2011).
Chen, W. & Kuo, T. A simple and rapid method for the preparation of gram negative bacterial genomic DNA. Nucleic Acids Res. 21, 2260 (1993).
Dumas, J. L., van Delden, C., Peron, K. & Köhler, T. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol. Lett. 254, 217–225 (2006).
Gaynes, R. & Edwards, J. R. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41, 848–854 (2005).
Karlowsky, J. A., Draghi, D. C., Jones, M. E., Thornsberry, C., Friedland, I. R. & Sahm, D. F. Surveillance for antimicrobial susceptibility among clinical isalates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2002. Antimicrob. Agents. Chemother. 47, 1681–1688 (2003).
Strateva, T., Ouzounova Raykova, V., Markova, B., Todorova, A., Marteva Proevska, Y. & Mitov, I. Problematic clinical isolates of Pseudomonas aeruginosa from the university hospitals in Sofia, Bulgaria: current status of antimicrobial resistance and prevailing resistance mechanisms. J. Med. Microbiol. 56, 956–963 (2007).
Sánchez-Romero, I. et al. Evolution of the antimicrobial resistance of Pseudomonas aeruginosa in Spain: Second National Study (2003). Rev. Esp. Quimioterap. 20, 222–229 (2007).
Obritsch, M. D., Fish, D. N., MacLaren, R. & Jung, R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 48, 4606–4610 (2004).
Savli, H. et al. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J. Med. Microbiol. 52, 1–6 (2003).
Kolayli, F. et al. Effect of carbapenems on the transcriptional expression of the oprD, OprM, OprN genes in Pseudomona aeruginosa. J. Med. Microbiol. 53, 915–920 (2004).
Poole, K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49, 479–487 2005.
Ozer, B., Tatman-Otkun, M., Memis, D. & Otkun, M. Characteristics of Pseudomonas aeruginosa isolates from intensive care unit. Cent. Eur. J. Med. 4, 156–163 (2009).
Masuda, N. et al. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM, efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3322–3327 (2000).
Maseda, H. et al. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48, 1320–1328 (2004).
Adewoye, L., Sutherland, A., Srikumar, R. & Poole, K. The MexR repressor of mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J. Bacteriol. 184, 4308–4312 (2002).
Quale, J., Bratu, S., Gupta, J. & Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 50, 1633–1641 (2006).
Kohler, T., Epp, S. F., Curty, L. K. & Pechere, J. C. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181, 6300–6305 (1999).
Livermore, D M. Interplay of impermeability and chromosomal beta lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36, 2046–2048 (1992).
Sakyo, S., Tomita, H., Tanimoto, K., Fujimoto, S. & Ike, Y. Potency of carbapenems for the prevention of carbapenem-resistant mutants of Pseudomonas aeruginosa. J. Antibiot. 59, 220–228 (2006).
Kim, J. Y. et al. Occurrence and mechanisms of amikacin resistance and its association with beta-lactamases in Pseudomonas aeruginosa: a Korean nationwide study. J. Antimicrob. Chemother. 62, 479–483 (2008).
Acknowledgements
This work was granted by Mustafa Kemal University Scientific Research Projects (BAP 08T 1701).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozer, B., Duran, N., Onlen, Y. et al. Efflux pump genes and antimicrobial resistance of Pseudomonas aeruginosa strains isolated from lower respiratory tract infections acquired in an intensive care unit. J Antibiot 65, 9–13 (2012). https://doi.org/10.1038/ja.2011.102
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2011.102
Keywords
This article is cited by
-
MIC score, a new tool to compare bacterial susceptibility to antibiotics application to the comparison of susceptibility to different penems of clinical strains of Pseudomonas aeruginosa
The Journal of Antibiotics (2016)
-
Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients
The Journal of Antibiotics (2015)
-
Emergence of antibiotic-resistant extremophiles (AREs)
Extremophiles (2012)