Abstract
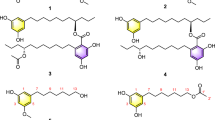
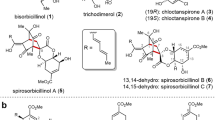
Two new acid sorbicillin analogues, (E)-6-(2,4-dihydroxyl-5-methylphenyl)-6-oxo-2-hexenoic acid (1) and 6-(2,4-dihydroxyl-5-methylphenyl)-6-oxohexanoic acid (2), together with 12 known compounds, were isolated from a saline lands-derived fungus Trichoderma sp. The structures of the new compounds were established by interpretation of their spectroscopic data. Their cytotoxic effects on P388 and HL-60 cell lines were preliminarily evaluated by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) method. Furthermore, the new compounds exhibited weak radical scavenging activity against DPPH (1,1-diphenyl-2-picrylhydrazyl).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Verma, M., Brar, S. K., Tyagi, R. D., Surampalli, R. Y. & Valero, J. R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control Biochem. Eng. J. 37, 1–20 (2007).
Montero, M., Sanz, L., Rey, M., Monte, E. & Llobell, A. BGN16.3, a novel acidic β-1,6-glucanase from mycoparasitic fungus Trichoderma harzianum CECT 2413. FEBS J 272, 3441–3448 (2005).
Ruiz, N. et al. New Trichobrachins, 11-residue peptaibols from a marine strain of Trichoderma longibrachiatum. Peptides 28, 1351–1358 (2007).
Macias, F. A. et al. Bioactive carotanes from Trichoderma virens. J. Nat. Prod. 63, 1197–1200 (2000).
Abe, N., Sugimoto, O., Hirota, A. & Murata, T. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 62, 661–666 (1998).
Abe, N., Murata, T. & Hirota, A. Novel oxidized sorbicillin dimmers with 1,1-diphenyl-2-picryldrazyl-radical scavenging activity from a fungus. Biosci. Biotechnol. Biochem. 62, 2120–2126 (1998).
Abe, N., Yamamoto, K. & Hirota, A. Novel fungal metabolites, demethylsorbicillin and oxosorbicillinol, isolated from Trichoderma sp. USF-2690. Biosci. Biotechnol. Biochem. 64, 620–622 (2000).
Du, L. et al. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem. Pharm. Bull. 57, 220–223 (2009).
Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province, China (No.Y2008B17). The working strain, Trichoderma sp JH8, was identified by Dr Tianjiao Zhu (Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China.). We appreciate Professor Qianqun Gu, Ocean University of China, for HRESIMS measurements and beneficial discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Liu, W., Huang, Y. et al. Two acid sorbicillin analogues from saline lands-derived fungus Trichoderma sp.. J Antibiot 64, 645–647 (2011). https://doi.org/10.1038/ja.2011.54
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2011.54
Keywords
This article is cited by
-
Endophytic fungi from Passiflora incarnata: an antioxidant compound source
Archives of Microbiology (2020)
-
Constitutive hyperproduction of sorbicillinoids in Trichoderma reesei ZC121
Biotechnology for Biofuels (2018)
-
Diphenyl derivatives from coastal saline soil fungus Aspergillus iizukae
Archives of Pharmacal Research (2015)