Abstract
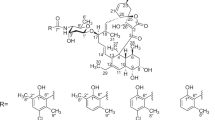
Nomimicin (1), a new spirotetronate-class polyketide, was isolated from the culture broth of an actinomycete of the genus Actinomadura. Its structure was established by spectroscopic methods, and the absolute configuration was determined by a combination of NOESY experiment, J-based configuration analysis and the modified Mosher method. Nomimicin (1) showed antimicrobial activity against Micrococcus luteus, Candida albincans and Kluyveromyces fragilis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Butler, M. S. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 25, 475–516 (2008).
Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 58, 1–26 (2005).
Smith, A. L. & Nicolaou, K. C. The enediyne antibiotics. J. Med. Chem. 39, 2103–2117 (1996).
Walsh, T. J. & Giri, N. Pradimicins: a novel class of broad-spectrum antifungal compounds. Eur. J. Clin. Microbiol. Infect. Dis. 16, 93–97 (1997).
Dirlam, J. P. et al. CP-84,657, a potent polyether anticoccidial related to portmicin and produced by Actinomadura sp. J. Antibiot. 43, 668–679 (1990).
Igarashi, Y. et al. Brartemicin, an inhibitor of tumor cell invasion from the actinomycete Nonomuraea sp. J. Nat. Prod 72, 980–982 (2009).
Igarashi, Y. et al. Maklamicin, an antibacterial polyketide from an endophytic Micromonospora sp. J. Nat. Prod. 74, 670–674 (2011).
Matsumori, N., Kaneno, D., Murata, M., Nakamura, H. & Tachibana, K. Stereochemical determination of acyclic structures based on carbon–proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 64, 866–876 (1999).
Furihata, K. & Seto, H. J-Resolved HMBC, a new NMR technique for measuring heteronuclear long-range coupling constants. Tetrahedron Lett. 40, 6271–6275 (1999).
Furihata, K., Tashiro, M. & Seto, H. Selective J-resolved HMBC, an efficient method for measuring heteronuclear long-range coupling constants. Magn. Reson. Chem. 47, 814–818 (2009).
Ohtani, I., Kusumi, T., Kashman, Y. & Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 113, 4092–4096 (1991).
Konno, K., Fujishima, T., Liu, Z. & Takayama, H. Determination of absolute configuration of 1,3-diols by the modified Mosher’s method using their di-MTPA esters. Chirality 13, 72–80 (2002).
Momose, I. et al. Decatromicins A and B, new antibiotics produced by Actinomadura sp. MK73-NF4. II. Structure determination. J. Antibiot. 52, 787–796 (1999).
Park, H-R., Chijiwa, S., Furihata, K., Hayakawa, Y. & Shin-ya, K. Relative and absolute configuration of versipelostatin, a down-regulator of molecular chaperone GRP78 expression. Org. Lett. 9, 1457–1460 (2007).
Mallams, A. K., Puar, M. S., Rossman, R. R., McPhail, A. T. & Macfarlane, R. D. Kijanimicin. 2. Structure and absolute stereochemistry of kijanimicin. J. Am. Chem. Soc. 103, 3940–3943 (1981).
Igarashi, Y. et al. Abyssomicin I, a modified polycyclic polyketide from Streptomyces sp. CHI39. J. Nat. Prod. 73, 1943–1946 (2010).
Fukuda, T. et al. Marianins A and B, prenylated phenylpropanoids from Mariannaea camptospora. J. Nat. Prod. 74, 1327–1330 (2011).
Acknowledgements
We acknowledge Dr T Okuda and Ms Y Sudoh at Tamagawa University for assistance with antimicrobial assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Igarashi, Y., Iida, T., Oku, N. et al. Nomimicin, a new spirotetronate-class polyketide from an actinomycete of the genus Actinomadura. J Antibiot 65, 355–359 (2012). https://doi.org/10.1038/ja.2012.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2012.30
Keywords
This article is cited by
-
Development of a drug discovery approach from microbes with a special focus on isolation sources and taxonomy
The Journal of Antibiotics (2023)
-
Promising bioactive compounds from the marine environment and their potential effects on various diseases
Journal of Genetic Engineering and Biotechnology (2022)
-
Actinomadura decatromicini sp. nov., isolated from mountain soil in Thailand
The Journal of Antibiotics (2021)
-
Structure elucidation and in silico docking studies of a novel furopyrimidine antibiotics synthesized by endolithic bacterium Actinomadura sp. AL2
World Journal of Microbiology and Biotechnology (2017)
-
Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061
Applied Microbiology and Biotechnology (2013)