Abstract
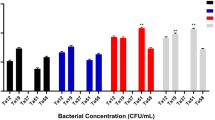
Two antimicrobial peptides (AMPs), named La47 and Css54, were isolated from the venom of the spider Lachesana sp. and from the scorpion Centruroides suffusus suffusus, respectively. The primary structures of both La47 and Css54 were determined using N-terminal sequencing and mass spectrometry. La47 is identical to the AMP latarcin 3a obtained previously from the venom of the spider Lachesana tarabaevi, but the primary structure of Css54 is unique having 60% identities to the AMP ponericin-W2 from the venom of the ant Pachycondyla goeldii. Both La47 and Css54 have typical α-helix secondary structures in hydrophobic mimicking environments. The biological activities of both La47 and Css54 were compared with the AMP Pin2 isolated from the venom of the scorpion Pandinus imperator. La47 has lower antimicrobial and hemolytic activities compared with Css54 and Pin2. In addition, La47 and Pin2 were evaluated in the presence of the commercial antibiotics, chloramphenicol, ampicillin, novobiocin, streptomycin and kanamycin. Interestingly, the best antimicrobial combinations were obtained with mixtures of La47 and Pin2 with the antibiotics chloramphenicol, streptomycin and kanamycin, respectively. Furthermore, the novel peptide Css54 was evaluated in the presence of antibiotics used for the treatment of tuberculosis, isoniazid, rifampicin, pyrazinamide and ethambutol. Although the mixtures of Css54 with isoniazid, pyrazinamide or ethambutol inhibit the growth of Staphylococcus aureus, the best effect was found with rifampicin. Overall, these data show a motivating outlook for potential clinical treatments of bacterial infections using AMPs and commercial antibiotics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jovetic, S., Zhu, Y., Marcone, G. L., Marinelli, F. & Tramper, J. Beta-Lactam and glycopeptide antibiotics: first and last line of defense? Trends. Biotechnol. 28, 596–604 (2010).
Pallecchi, L., Bartoloni, A., Paradisi, F. & Rossolini, G. M. Antibiotic resistance in the absence of antimicrobial use: mechanisms and implications. Expert. Rev. Anti. Infect Ther. 6, 725–732 (2008).
Walsh, C. Where will new antibiotics come from? Nat. Rev. Microbiol. 1, 65–70 (2003).
Lambert, R. J. & Lambert, R. A model for the efficacy of combined inhibitors. J. Appl. Microbiol. 95, 734–743 (2003).
Linden, P. K. Treatment options for vancomycin-resistant enterococcal infections. Drugs 62, 425–441 (2002).
Maróti, G., Kereszt, A., Kondorosi, E. & Mergaert, P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 162, 363–374 (2011).
Brogden, N. K. & Brogden, K. A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J Antimicrob. Agents 38, 217–225 (2011).
Steinstraesser, L., Kraneburg, U., Jacobsen, F. & Al-Benna, S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 216, 322–333 (2011).
Villegas, E. & Corzo, G. Pore-forming peptides from spiders. Toxin Rev. 24, 345–357 (2005).
Corzo, G. et al. Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem. J 359, 35–45 (2001).
Kuhn-Nentwig, L. Antimicrobial and cytolytic peptides of venomous arthropods. Cell Mol. Life Sci. 60, 2651–2668 (2003).
Ramirez-Carreto, S. et al. Gene cloning and functional characterization of four novel antimicrobial-like peptides from scorpions of the family Vaejovidae Peptides. Peptides 34, 290–295 (2012).
Torres-Larios, A., Gurrola, G. B., Zamudio, F. Z. & Possani, L. D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 267, 5023–5031 (2000).
Yan, L. & Adams, M. E. Lycotoxins, antimicrobial peptides from venom of the wolf spider Lycosa carolinensis. J. Biol. Chem. 273, 2059–2066 (1998).
Zeng, X. C., Corzo, G. & Hahin, R. Scorpion venom peptides without disulfide bridges. IUBMB Life 57, 13–21 (2005).
Conde, R., Zamudio, F. Z., Rodriguez, M. H. & Possani, L. D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett 471, 165–168 (2000).
Corzo, G. et al. Oxyopinins, large amphipathic peptides isolated from the venom of the wolf spider Oxyopes kitabensis with cytolytic properties and positive insecticidal cooperativity with spider neurotoxins. J. Biol. Chem. 277, 23627–23637 (2002).
Belokoneva, O. S. et al. Pore formation of phospholipid membranes by the action of two hemolytic arachnid peptides of different size. Biochim. Biophys. Acta. 1664, 182–188 (2004).
Belokoneva, O. S., Villegas, E., Corzo, G., Dai, L. & Nakajima, T. The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine-to-sphingomyelin ratio in lipid bilayers. Biochim. Biophys. Acta. 1617, 22–30 (2003).
Keymanesh, K., Soltani, S. & Sardari, S. Application of antimicrobial peptides in agriculture and food industry. World J Microbiol Biotechnol. 25, 933–944 (2009).
Sonnichsen, F. D., Van Eyk, J. E., Hodges, R. S. & Sykes, B. D. Effect of trifluoroethanol on protein secondary structure: an NMR and CD study using a synthetic actin peptide. Biochemistry 31, 8790–8798 (1992).
Eliopoulos, G. M., Moellering, R. C. Antimicrobial combinations. Antibiotics in laboratory medicine (ed Lorian, V. ) 3rd edn. Baltimore: The Williams & Wilkins Co (1991).
White, R. L., Burgess, D. S., Manduru, M. & Bosso, J. A. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 40, 1914–1918 (1996).
Kozlov, S. A. et al. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J. Biol. Chem. 281, 20983–20992 (2006).
Orivel, J. et al. Ponericins, new antibacterial and insecticidal peptides from the venom of the ant Pachycondyla goeldii. J. Biol. Chem. 276, 17823–17829 (2001).
Park, J. M., Jung, J. E. & Lee, B. J. Antimicrobial peptides from the skin of a Korean frog. Rana rugosa. Biochem. Biophys. Res. Commun. 205, 948–954 (1994).
Morikawa, N., Hagiwara, K. & Nakajima, T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 189, 184–190 (1992).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Liu, Y. et al. Selection of rifampicin-resistant Staphylococcus aureus during tuberculosis therapy: concurrent bacterial eradication and acquisition of resistance. J. Antimicrob. Chemother. 56, 1172–1175 (2005).
Goraya, J., Knoop, F. C. & Conlon, J. M. Ranateurin 1T: an antimicrobial peptide isolated from the skin of the frog, Rana temporaria. Peptides 20, 159–163 (1999).
Nakajima, T. Pharmacological biochemistry of Vespid venoms In Venoms of Hymenoptera (ed Piek T., ) Academic Press: London), 1986.
Steiner, H., Andreu, D. & Merrifield, R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim. Biophys. Acta. 939, 260–266 (1988).
Iwai, H., Nakajima, Y., Natori, S., Arata, Y. & Shimada, I. Solution conformation of an antibacterial peptide, sarcotoxin IA, as determined by 1H-NMR. Eur. J. Biochem. 217, 639–644 (1993).
Sitaram, N. & Nagaraj, R. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim. Biophys. Acta. 1462, 29–54 (1999).
Wencewicz, T. A., Möllmann, U., Long, T. E. & Miller, M. J. Is drug release necessary for antimicrobial activity of siderophore-drug conjugates Syntheses and biological studies of the naturally occurring salmycin ‘‘Trojan Horse’’ antibiotics and synthetic desferridanoxamine-antibiotic conjugates. Biometals 22, 633–648 (2009).
Acknowledgements
The mass spectrometry determination conducted by Dr Fernando Zamudio is greatly acknowledged. We also acknowledge QBP Ma. Rocio Patiño Maya from Instituto de Química—UNAM for CD spectra data acquisition. The patent filling advice from MSc Martin Patiño and MBA Mario Trejo is also greatly acknowledged. Francia García was recipient of an MSc scholarship (#215024) from CONACyT. This work was partially supported by grants from Laboratorios Silanes SA de CV and CONACyT 153606.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, F., Villegas, E., Espino-Solis, G. et al. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J Antibiot 66, 3–10 (2013). https://doi.org/10.1038/ja.2012.87
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2012.87
Keywords
This article is cited by
-
Antimicrobial Activity of Two Novel Venoms from Saudi Arabian Scorpions (Leiurus quinquestriatus and Androctonus crassicauda)
International Journal of Peptide Research and Therapeutics (2020)
-
Marine Antimicrobial Peptides: A Promising Source of New Generation Antibiotics and Other Bio-active Molecules
International Journal of Peptide Research and Therapeutics (2019)