Abstract
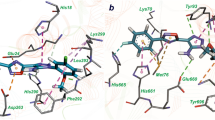
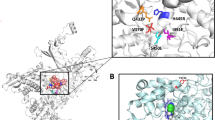
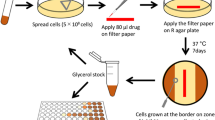
Capuramycin (1) and its analogs are strong translocase I (MurX/MraY) inhibitors. In our structure–activity relationship studies of capuramycin analogs against Mycobacterium tuberculosis (Mtb), we observed for the first time that a capuramycin analog, UT-01320 (3) killed nonreplicating (dormant) Mtb at low concentrations under low oxygen conditions, whereas selective MurX inhibitors killed only replicating Mtb under aerobic conditions. Interestingly, 3 did not exhibit MurX enzyme inhibitory activity even at high concentrations, however, 3 inhibited bacterial RNA polymerases with the IC50 values of 100–150 nM range. A new RNA polymerase inhibitor 3 displayed strong synergistic effects with a MurX inhibitor SQ 641 (2), a promising preclinical tuberculosis drug.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nakanjako D et al. Tuberculosis and human immunodeficiency virus co-infections and their predictors at a hospital-based HIV/AIDS clinic in Uganda. Int. J. Tuberc. Lung Dis. 14, 1621–1628 (2010).
Diedrich CR, Flynn JL HIV-1/Mycobacterium tuberculosis co-infection immunology: How does HIV-1 exacerbate tuberculosis? Infect. Immun. 79, 1407–1417 (2011).
Chien JY et al. Decline in rates of acquired multidrug-resistant tuberculosis after implementation of the directly observed therapy, short course (DOTS) and DOTS-Plus programmes in Taiwan. J. Antimicrob. Chemother. 68, 1910–1916 (2013).
Ranjbar S et al. HIV-1 Replication is differentially regulated by distinct clinical strains of Mycobacterium tuberculosis. PLoS ONE 4, e6116 (2009).
Connolly LE, Edelstein PH, Ramakrishnan L Why is long-term therapy required to cure tuberculosis? PLoS Med. 4, 435–442 (2003).
Miranda MS, Breiman A, Allain S, Deknuydt F, Altare F The tuberculous granuloma: an unsuccessful host defense mechanism providing a safety shelter for the bacteria? Clin. Develop. Immun. 2012, 1–14 (2012).
Wayne LG, Hayes LG An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64, 2062–2069 (1996).
Claudia S et al. Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 54, 4150–4158 (2010).
van Heijenoort J Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 71, 620–635 (2007).
Hett EC, Rubin EJ Bacterial growth and cell division: a Mycobacterial perspective. Microbiol. Mol. Biol. Rev. 72, 126–156 (2008).
Barry CE, Blanchard JS The chemical biology of new drugs in development for tuberculosis. Curr. Opin. Chem. Biol. 14, 456–466 (2010).
Reddy VM, Einck L, Nacy CA In vitro antimycobacterial activity of capuramycin analogs. Antimicro. Agents Chemother. 52, 719–721 (2008).
Kurosu M, Li K Synthetic studies towards the identification of novel capuramycin analogs with antimycobacterial activity. Heterocycles 77, 217–225 (2009).
Kurosu M, Li K, Crick DC A concise synthesis of capuramycin. Org. Lett. 11, 2393–2396 (2009).
Wang Y, Siricilla S, Aleiwi BA, Kurosu M Improved synthesis of capuramycin and its analogues. Chem. Eur. J. 19, 13847–13858 (2013).
Koga T et al. Activity of capuramycin analogs against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellular in vitro and in vivo. J. Antimicro. Chemother. 54, 755–760 (2004).
Nikonenko BV et al. Activity of SQ641, a capuramycin analog, in a murine model of tuberculosis. Antimicrob. Agents Chemother. 53, 3138–3139 (2009).
Dubuisson T et al. In vitro antimicrobial activities of capuramycin analogues against non-tuberculous mycobacteria. J. Antimicrob. Chemother. 65, 2590–2597 (2010).
Bogatcheva E et al. Chemical modification of capuramycins to enhance antibacterial activity. J. Antimicrob. Chemother. 66, 578–587 (2011).
Cho SH et al. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 51, 1380–1358 (2007).
Siricilla S, Mitachi K, Skorupinska-Tudek K, Swiezewska E, Kurosu M Biosynthesis of a water-soluble lipid I analogue and a convenient assay for translocase I. Anal. Biochem. 461, 36–35 (2014).
Wayne LG, Sramek HA Antigenic differences between extracts of actively replicating and synchronized resting cells of Mycobacterium tuberculosis. Infect. Immun. 24, 363–370 (1979).
Gengenbacher M, Rao SPS, Pethe K, Dick T Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology 156, 81–87 (2010).
Hsieh MH, Chen MY, Victor LY, Chow JW Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 16, 343–349 (1993).
Meletiadis J, Pournaras S, Roilides E, Walsh TJ Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, monte carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 54, 602–609 (2010).
Debnath J et al. Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis. J. Med. Chem. 55, 3739–3755 (2012).
Levin ME, Hatfull GF Mycobacterium smegmatis RNA polymerase: DNA supercoiling, action of rifampicin and mechanism of rifampicin resistance. Mol. Microbiol. 8, 277–285 (1993).
Siegmund V, Santner T, Micura R, Marx A Screening mutant libraries of T7 RNA polymerase for candidates with increased acceptance of 2'-modified nucleotides. Chem. Commun. 48, 9870–9872 (2012).
Kuhlman P, Duff HL, Galant A A fluorescence-based assay for multisubunit DNA-dependent RNA polymerases. Analy. Biochem. 324, 183–190 (2004).
Onodera K, Kawasaki T, Kamijo S Discovery of novel antimicrobial agents targeting the bacterial RNA polymerase by high-throughput virtual screening. Chem-Bio. Informat. J. 11, 52–62 (2011).
Boshoff HIM, Barry CE Tuberculosis - metabolism and respiration in the absence of growth. Nature Rev. Microbiol. 3, 70–80 (2005).
Ishizaki Y et al. Inhibition of the first step in synthesis of the Mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45. J. Biol. Chem. 288, 30309–30319 (2013).
Engohang-Ndong J Antimycobacterial drugs currently in phase II clinical trials and preclinical phase for tuberculosis treatment. Expert Opin. Investig. Drugs. 21, 1789–1800 (2012).
Campbell EA et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104, 901–912 (2001).
Snewin VA et al. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 67, 4586–4593 (1999).
Kurosu M, Mahapatra S, Narayanasamy P, Crick DC Chemoenzymatic synthesis of park’s nucleotide: toward the development of high-throughput screening for MraY inhibitors. Tetrahedron Lett. 48, 799–803 (2007).
Li K, Kurosu M Synthetic studies on Mycobacterium tuberculosis specific fluorescent park’s nucleotide probe. Heterocycles 76, 455–469 (2008).
Mitachi K, Mohan P, Siricilla S, Kurosu M One-pot protection-glycosylation reactions for synthesis of lipid II analogues. Chem. A. Eur. J. 20, 4554–4558 (2014).
Mandana R, Marie-Antoinette L, Peter S, Mamadou D Breaking down the wall: Fractionation of mycobacteria. J. Microbiol. Methods 68, 32–39 (2007).
Bouhss A, Crouvoisier M, Blanot D, Mengin-Lecreulx D Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 279, 29974–29980 (2004).
Burgess RR, Jendrisak JJ A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving polymin P precipitation and DNA-cellulose chromatography. Biochemistry 14, 4634–4638 (1975).
Ohrt C, Willingmyre GD, Lee P, Knirsch C, Milhous W Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 46, 2518–2524 (2002).
Acknowledgements
The National Institutes of Health is greatly acknowledged for financial support of this work (AI084411). We also thank University of Tennessee for generous financial support. NMR data were obtained on instruments supported by the NIH Shared Instrumentation Grant. The following reagent was obtained through BEI Resources, NIAID, NIH: M. tuberculosis, strain H37Rv and gamma-irradiated M. tuberculosis, NR-14819. The authors gratefully acknowledge Drs William Clemons (California Institute Technology) and Crick (Colorado State University) for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siricilla, S., Mitachi, K., Wan, B. et al. Discovery of a capuramycin analog that kills nonreplicating Mycobacterium tuberculosis and its synergistic effects with translocase I inhibitors. J Antibiot 68, 271–278 (2015). https://doi.org/10.1038/ja.2014.133
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2014.133
This article is cited by
-
Chemical logic of MraY inhibition by antibacterial nucleoside natural products
Nature Communications (2019)
-
Winners of the 2016 JA Medals for excellence
The Journal of Antibiotics (2017)
-
Sansanmycin natural product analogues as potent and selective anti-mycobacterials that inhibit lipid I biosynthesis
Nature Communications (2017)