Abstract
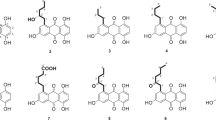
Two new hydroanthraquinones, paradictyoarthrins A (1) and B (2), were isolated from the mangrove-derived fungus Paradictyoarthrinium diffractum BCC 8704. Structures of the new compounds were elucidated by analyses of the NMR spectroscopic and mass spectrometry data. The absolute configuration of 1 was determined by X-ray crystallography. These compounds exhibited cytotoxic activities.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rateb, M. E. & Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 28, 290–344 (2011).
Blunt, J. W., Copp, B. R., Keyzers, R. A. & Munro, M. H. G. Marine natural products. Nat. Prod. Rep. 31, 160–258 (2014).
Weber, H. A. & Gloer, J. B. The preussomerins: novel antifungal metabolites from the coprophilous fungus Preussia isomera Cain. J. Org. Chem. 56, 4355–4360 (1991).
Dong, J. Y. et al. Ymf 1029A–E, preussomerin analogues from fresh-water-derived fungus YMF 1.01029. J. Nat. Prod. 71, 952–956 (2008).
Coombe, R. G., Jacobs, J. J. & Watson, T. R. Metabolites of some Alternaria species. The structures of altenusin and dehydroaltenusin. Aust. J. Chem. 23, 2343–2351 (1970).
Ondeyka, J. et al. Isolation, structure elucidation, and biological activity of altersolanol P using Staphylococcus aureus fitness test based genome-wide screening. J. Nat. Prod. 77, 497–502 (2014).
Jackman, L. M. & Sternhell, S. Applications of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry 2nd edn, 88–92 (Pergamon Press: Oxford, (1969).
Zheng, C.-G. et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 75, 189–197 (2012).
Xu, J. et al. Tetrahydrobostrycin and 1-deoxytetrahydrobostrycin, two new hexahydroanthraquinone dimers, from a marine-derived fungus Aspergillus sp. J. Antibiot. 61, 415–419 (2008).
Yang, K.-L. et al. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 75, 935–941 (2012).
Alvi, K. A. & Rabenstein, J. Auxarthrol A and auxarthrol B: two new tetrahydroanthraquinones from Auxarthron umbrinum. J. Ind. Microbiol. Biotechnol. 31, 11–15 (2004).
Cai, Y.-S., Guo, Y.-W. & Krohn, K. Structure, bioactivities, biosynthetic relationships and chemical synthesis of the spirodioxynaphthalenes. Nat. Prod. Rep. 27, 1840–1870 (2010).
Altomare, A. et al. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 32, 115–119 (1999).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
O’Brien, J., Wilson, I., Orton, T. & Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426 (2000).
Changsen, C., Franzblau, S. G. & Palittapongarnpim, P. Improved green fluorescent protein reporter gene-based microplate screening for antituberculosis compounds by utilizing an acetamidase promoter. Antimicrob. Agents Chemother. 47, 3682–3687 (2003).
Acknowledgements
Financial support from the National Center for Genetic Engineering and Biotechnology (BIOTEC) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Isaka, M., Chinthanom, P., Rachtawee, P. et al. Cytotoxic hydroanthraquinones from the mangrove-derived fungus Paradictyoarthrinium diffractum BCC 8704. J Antibiot 68, 334–338 (2015). https://doi.org/10.1038/ja.2014.153
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2014.153
This article is cited by
-
Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa
Fungal Diversity (2020)