Abstract
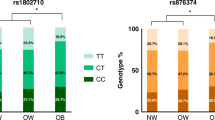
Leptin plays an important role in regulating adipose-tissue mass. Leptin controls energy balance and food intake through the leptin receptor in the hypothalamus of the brain, which suggests that some polymorphisms of the leptin receptor gene (LEPR) might contribute to obesity or obesity-related diseases. In an effort to identify genetic polymorphisms in a potential candidate gene for obesity and type 2 diabetes mellitus (T2DM) in the Korean population, we have sequenced the LEPR gene. Thirty-five sequence variants were identified (including 9 novel polymorphisms): 1 single nucleotide polymorphism (SNP) in the promoter region, 1 SNP in the 5′ UTR, 8 SNPs in exons (3 non-synonymous SNPs), 23 SNPs in introns, 1 ins/del in the 3′ UTR, and 1 SNP in the 3′ downstream region. To investigate possible association of LEPR polymorphisms with body mass index (BMI) and the risk of T2DM, we genotyped for 11 polymorphisms in the Korean population (n=1,463). Using statistical analyses, no significant associations between the genetic polymorphisms in the LEPR gene and the risk of T2DM were detected. However, one non-synonymous SNP in exon 3, +5193G>A (Arg109Lys), showed marginal association with BMI (P=0.02) and gene dose-dependent genetic effects were observed. The present study provides information about additional genetic polymorphisms in LEPR and positive associations of those polymorphisms with BMI in the Korean population.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aiston S, Agius L (1999) Leptin enhances glycogen storage in hepatocytes by inhibition of phosphorylase and exerts an additive effect with insulin. Diabetes 48:15–20
Berti L, Kellerer M, Capp E, Haring HU (1997) Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia 40:606–609
Bouchard C (1994) Genetics of obesity: overview and research directions. In: Bouchard C (ed) The genetics of obesity. CRC Press
Ceddia RB, William WN Jr, Curi R (1999) Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord 23:75–82
Chiu KC, Chu A, Chuang LM, Saad MF (2004) Association of leptin receptor polymorphism with insulin resistance. Eur J Endocrinol 150:725–729
Cohen B, Novick D, Rubinstein M (1996) Modulation of insulin activities by leptin. Science 274:1185–1188
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M (1997) Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46:313–316
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770
Fukuda H, Iritani N, Sugimoto T, Ikeda H (1999) Transcriptional regulation of fatty acid synthase gene by insulin/glucose, polyunsaturated fatty acid and leptin in hepatocytes and adipocytes in normal and genetically obese rats. Eur J Biochem 260:505–511
Guizar-Mendoza JM, Amador-Licona N, Flores-Martinez SE, Lopez-Cardona MG, Ahuatzin-Tremary R, Sanchez-Corona J (2005) Association analysis of the Gln223Arg polymorphism in the human leptin receptor gene, and traits related to obesity in Mexican adolescents. J Hum Hypertens 19:341–346
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546
Heo M, Leibel RL, Fontaine KR, Boyer BB, Chung WK, Koulu M, Karvonen MK, et al. (2002) A meta-analytic investigation of linkage and association of common leptin receptor (LEPR) polymorphisms with body mass index and waist circumference. Int J Obes Relat Metab Disord 26:640–646
Kieffer TJ, Heller RS, Habener JF (1996) Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun 224:522–527
Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF (1997) Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 46:1087–1093
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161
Mantzoros CS (1999) The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med 130:671–680
Mantzoros CS, Flier JS (2000) Editorial: leptin as a therapeutic agent—trials and tribulations. J Clin Endocrinol Metab 85:4000–4002
Mattevi VS, Zembrzuski VM, Hutz MH (2002) Association analysis of genes involved in the leptin-signaling pathway with obesity in Brazil. Int J Obes Relat Metab Disord 26:1179–1185
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P (2000) Hypertension in obesity and the leptin receptor gene locus. J Clin Endocrinol Metab 85:3126–3131
Salopuro T, Pulkkinen L, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Laakso M, Uusitupa M (2005) Genetic variation in leptin receptor gene is associated with type 2 diabetes and body weight: the Finnish diabetes prevention study. Int J Obes 29:1245–1251
Santaniemi M, Ukkola O, Kesaniemi YA (2004) Tyrosine phosphatase 1B and leptin receptor genes and their interaction in type 2 diabetes. J Intern Med 256:48–55
Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA (2002) Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425–434
Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG (1997) Effects of leptin on insulin sensitivity in normal rats. Endocrinology 138:3395–3401
Takahashi-Yasuno A, Masuzaki H, Miyawaki T, Ogawa Y, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K (2003) Leptin receptor polymorphism is associated with serum lipid levels and impairment of cholesterol lowering effect by simvastatin in Japanese men. Diabetes Res Clin Pract 62:169–175
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271
The expert committee on the diagnosis and classification of diabetes mellitus (1997) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20:1183–1197
Van Rossum CT, Hoebee B, van Baak MA, Mars M, Saris WH, Seidell JC (2003) Genetic variation in the leptin receptor gene, leptin, and weight gain in young Dutch adults. Obes Res 11:377–386
Wauters M, Mertens I, Rankinen T, Chagnon M, Bouchard C, Van Gaal L (2001) Leptin receptor gene polymorphisms are associated with insulin in obese women with impaired glucose tolerance. J Clin Endocrinol Metab 86:3227–3232
Woods AJ, Stock MJ (1996) Leptin activation in hypothalamus. Nature 381:745
Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS (2001) The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab 86:4434–4439
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Acknowledgements
This work was supported by an intramural grant of the National Institute of Health, Korea, and a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (00-PJ3-PG6-GN07-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kyong Soo Park and Hyoung Doo Shin contributed equally to this work
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Park, K.S., Shin, H.D., Park, B.L. et al. Polymorphisms in the leptin receptor (LEPR)—putative association with obesity and T2DM. J Hum Genet 51, 85–91 (2006). https://doi.org/10.1007/s10038-005-0327-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1007/s10038-005-0327-8
Keywords
This article is cited by
-
Leptin receptor gene polymorphisms c.668A>G and c.1968G>C in Sudanese women with preeclampsia: a case-control study
BMC Medical Genetics (2020)
-
The influence of the rs1137101 genotypes of leptin receptor gene on the demographic and metabolic profile of normal Saudi females and those suffering from polycystic ovarian syndrome
BMC Women's Health (2019)
-
Obesity and microbiota: an example of an intricate relationship
Genes & Nutrition (2017)
-
Unravelling the effects of the environment and host genotype on the gut microbiome
Nature Reviews Microbiology (2011)
-
Leptin receptor (LEPR) SNP polymorphisms in HELLP syndrome patients determined by quantitative real-time PCR and melting curve analysis
BMC Medical Genetics (2010)