Abstract
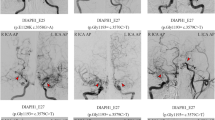
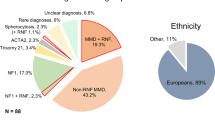
Moyamoya disease (MMD) is a disease pattern consisting of bilateral stenosis of the intracranial internal carotid arteries (ICA) accompanied by a network of abnormal collateral vessels that bypass the stenosis. Once symptomatic, insufficient cerebral blood flow or rupture of the fragile collaterals may cause stroke or hemorrhage, resulting in severe neurological dysfunction or death. The etiology of MMD is still unknown, although few associations with other diseases and environmental factors have been described. Strong regional differences in epidemiological data, as well as known familial cases, turned the focus to genetics for the insight into the disease's pathogenesis. Thus far, several reports have suggested specific genetic loci and individual genes as predisposing to MMD, but none have demonstrated reproducible results in independent cohorts. Small sample sizes, as well as a likely multifactorial origin, seem to be the most challenging tasks in identifying the disease-causing mechanisms. Once identified, susceptibility genes may allow preventive screening and a possible development of novel therapeutic options.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Takeuchi, K. & Shimizu, K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 9, 37–43 (1957).
Suzuki, J. & Takaku, A. Cerebrovascular ‘moyamoya’ disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 20, 288–299 (1969).
Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 99, S238 (1997).
Yonekawa, Y. & Taub, E. Moyamoya disease: status 1998. Neurologist. 5, 13–23 (1999).
Khan, N. & Yonekawa, Y. Moyamoya angiopathy in Europe: ‘The Zürich experience’. Stroke Rev. 9, 181–188 (2005).
Uchino, K., Johnston, S. C., Becker, K. J. & Tirschwell, D. L. Moyamoya disease in Washington state and California. Neurology. 65, 956–958 (2005).
Baba, T., Houkin, K. & Kuroda, S. Novel epidemiological features of moyamoya disease. Br. Med. J. 79, 900–904 (2008).
Wakai, K., Tamakoshi, A., Ohno, Y., Kawamura, T., Ikezaki, K. & Fukui, M. Epidemiology of spontaneous occlusion of the circle of Willis: results of national epidemiologic survey. The Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare Japan: Annual Report 1995 33–37 (1995).
Fukui, M. Current state of study on moyamoya disease in Japan. Surg. Neurol. 47, 138–143 (1997).
Ikezaki, K., Han, D. H., Kawano, T., Kinukawa, N. & Fukui, M. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke 28, 2513–2517 (1997).
Iwama, T. & Yoshimura, S. Present status of Moyamoya disease in Japan. Acta. Neurochir. (Wien). 103, 115–118 (2008).
Scott, R. M. & Smith, E. R. Moyamoya disease and Moyamoya syndrome. N. Engl. J. Med. 360, 1226–1237 (2009).
Ikezaki, K. Rational approach to treatment of Moyamoya disease in childhood. J. Child Neurol. 15, 350–356 (2000).
Khan, N., Schuknecht, B., Boltshauser, E., Capone, A., Buck, A., Imhof, H. et al. Moyamoya disease and Moyamoya syndrome: experience in Europe; choice of revascularisation procedures. Acta. Neurochir. (Wien). 145, 1061–1071 (2003).
Matsushima, Y., Aoyagi, M., Suzuki, R., Nariai, T., Shishido, T. & Hirakawa, K. Dual anastomosis for pediatric moyamoya patients using the anterior and the posterior branches of the superficial temporal artery. Nerv. Syst. Child. 18, 27–32 (1993).
Takagi, Y., Kikuta, K., Nozaki, K. & Hashimoto, N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol. Med. Chir. (Tokyo). 47, 1–4 (2007).
Ullrich, N. J., Robertson, R., Kinnamon, D. D., Scott, R. M., Kieran, M. W., Turner, C. D. et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 68, 932–938 (2007).
Yamada, H., Deguchi, K., Tanigawara, T., Takenaka, K., Nishimura, Y., Shinoda, J. et al. The relationship between Moyamoya disease and bacterial infection. Clin. Neurol. Neurosurg. 99, S221 (1997).
Fukuyama, S., Kanai, M. & Osawa, M. Clinical genetic analysis on the Moyamoya disease. The Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare Japan: Annual report 1990 53–59 (1990).
Osawa, M., Kanai, N., Kawai, M. & Fukuyama, Y. Clinical genetic study on the idiopathic occlusion of the circle of Willis. The research committee on spontaneous occlusion of the circle of Willis (Moyamoya disease) of the Ministry of Health and Welfare in Japan. Annual Report Tokyo, Japan: Ministry of Health and Welfare, Japan 147–152 (1992).
Yamauchi, T., Houkin, K., Tada, M. & Abe, H. Familial occurrence of moyamoya disease. Clin. Neurol. Neurosurg. 99, 159–164 (1997).
Nanba, R., Kuroda, S., Tada, M., Ishikawa, T., Houkin, K. & Iwasaki, Y. Clinical features of familial moyamoya disease. Childs Nerv. Syst. 22, 258–262 (2006).
Kuroda, S. & Houkin, K. Moyamoya disease: current concepts and future perspectives. Lancet. Neurol. 7, 1056–1066 (2008).
Smith, E. R. & Scott, R. M. Surgical management of moyamoya syndrome. Skull Base. 15, 15 (2005).
Inoue, T. K., Ikezaki, K., Sasazuki, T., Matsushima, T. & Fukui, M. Analysis of class II genes of human leukocyte antigen in patients with Moyamoya disease. Clin. Neurol. Neurosurg. 99, S229–S232 (1997).
Ikeda, H., Sasaki, T., Yoshimoto, T., Fukui, M. & Arinami, T. Mapping of a familial moyamoya disease gene to chromosome 3p24. 2-p26. Am. J. Hum. Genet. 64, 533–537 (1999).
Collod, G., Babron, M. C., Jondeau, G., Coulon, M., Weissenbach, J., Dubourg, O. et al. A second locus for Marfan syndrome maps to chromosome 3p24. 2-p25. Nat. Genet. 8, 264 (1994).
Latif, F., Tory, K., Gnarra, J., Yao, M., Duh, F. M., Orcutt, M. L. et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 260, 1317–1320 (1993).
Inoue, T. K., Ikezaki, K., Sasazuki, T., Ono, T., Kamikawaji, N., Matsushima, T. et al. DNA typing of HLA in the patients with moyamoya disease. J. Hum. Genet. 42, 507–515 (1997).
Inoue, T. K., Ikezaki, K., Sasazuki, T., Matsushima, T. & Fukui, M. Linkage analysis of moyamoya disease on chromosome 6. J. Child Neurol. 15, 179–182 (2000).
Yamauchi, T., Tada, M., Houkin, K., Tanaka, T., Nakamura, Y., Kuroda, S. et al. Linkage of familial moyamoya disease (spontaneous occlusion of the circle of willis) to chromosome 17q25. Stroke. 31, 930–935 (2000).
Xu, G. F., O’Connell, P., Viskochil, D., Cawthon, R., Robertson, M., Culver, M. et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62, 599–608 (1990).
Barrall, J. L. & Summers, C. G. Ocular ischemic syndrome in a child with moyamoya disease and neurofibromatosis. Surv. Ophthalmol. 40, 500–504 (1996).
Edwards Brown, M. K. & Quets, J. P. Midwest experience with moyamoya disease. Clin. Neurol. Neurosurg. 99, 36–38 (1997).
Woody, R. C., Perrot, L. J. & Beck, S. A. Neurofibromatosis cerebral vasculopathy in an infant: clinical, neuroradiographic, and neuropathologic studies. Fetal. Pediatr. Pathol. 12, 613–619 (1992).
Kwong, K. L. & Wong, Y. C. Moyamoya disease in a child with neurofibromatosis type-1. J. Paediatr. Child Health. 35, 108–109 (1999).
Aoyagi, M., Ogami, K., Matsushima, Y., Shikata, M., Yamamoto, M. & Yamamoto, K. Human leukocyte antigen in patients with Moyamoya disease. Stroke. 26, 415–417 (1995).
Han, H., Pyo, C., Yoo, D., Huh, P., Cho, K. & Kim, D. Associations of Moyamoya patients with HLA class I and class II alleles in the Korean population. J. Korean Med. Sci. 18, 876–880 (2003).
Sakurai, K., Horiuchi, Y., Ikeda, H., Ikezaki, K., Yoshimoto, T., Fukui, M. et al. A novel susceptibility locus for moyamoya disease on chromosome 8q23. J. Hum. Genet. 49, 278–281 (2004).
Nanba, R., Tada, M., Kuroda, S., Houkin, K. & Iwasaki, Y. Sequence analysis and bioinformatics analysis of chromosome 17q25 in familial moyamoya disease. Childs Nerv. Syst. 21, 62–68 (2005).
Kang, H. S., Kim, S. K., Cho, B. K., Kim, Y. Y., Hwang, Y. S. & Wang, K. C. Single nucleotide polymorphisms of tissue inhibitor of metalloproteinase genes in familial moyamoya disease. Neurosurgery. 58, 1074–1080 (2006).
Johnson, C. & Galis, Z. S. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler. Thromb. Vasc. Biol. 24, 54 (2004).
Dollery, C. M., McEwan, J. R., Wang, M., Sang, Q. A., Liu, Y. E. & Shi, Y. E. TIMP-4 is regulated by vascular injury in rats. Ann. N. Y. Acad. Sci. 84, 498–504 (1999).
Hasenstab, D., Forough, R. & Clowes, A. W. Plasminogen activator inhibitor type 1 and tissue inhibitor of metalloproteinases-2 increase after arterial injury in rats. Circ. Res. 80, 490–496 (1997).
Shi, Y., Patel, S., Niculescu, R., Chung, W., Desrochers, P. & Zalewski, A. Role of matrix metalloproteinases and their tissue inhibitors in the regulation of coronary cell migration. Arterioscler. Thromb. Vasc. Biol. 19, 1150–1155 (1999).
Paszkowiak, J. J. & Dardik, A. Arterial wall shear stress: observations from the bench to the bedside. Vasc. Endovascular Surg. 37, 47 (2003).
Mineharu, Y., Takenaka, K., Yamakawa, H., Inoue, K., Ikeda, H., Kikuta, K. I. et al. Inheritance pattern of familial moyamoya disease: autosomal dominant mode and genomic imprinting. J. Neurol. Neurosurg. Psychiatry. 77, 1025–1029 (2006).
Mineharu, Y., Liu, W., Inoue, K., Matsuura, N., Inoue, S., Takenaka, K. et al. Autosomal dominant moyamoya disease maps to chromosome 17q25.3. Neurology. 70, 2357–2363 (2008).
Guo, D.- C., Papke, C. L., Tran-Fadulu, V., Regalado, E. S., Avidan, N., Johnson, R. J. et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 84, 617–627 (2009).
Hong, S., Wang, K., Kim, S., Cho, B. & Park, M. Association of HLA-DR and-DQ genes with familial moyamoya disease in Koreans. J. Korean Neurosurg. Soc. 46, 558–563 (2009).
Liu, W., Hashikata, H., Inoue, K., Matsuura, N., Mineharu, Y., Kobayashi, H. et al. A rare Asian founder polymorphism of RAPTOR may explain the high prevalence of moyamoya disease among East Asians and its low prevalence among Caucasians. Env. Health Prev. Med. 1–11 (2009).
Roder, C., Peters, V., Kasuya, H., Nishizawa, T., Takehara, Y., Berg, D. et al. Polymorphisms in TGFB1 and PDGFRB are associated with Moyamoya disease in European patients. Acta. Neurochir. (Wien). (2010). (e-pub ahead of print 23 June 2010; DOI 10.1007/s00701-00010-00711-00709).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roder, C., Nayak, N., Khan, N. et al. Genetics of Moyamoya disease. J Hum Genet 55, 711–716 (2010). https://doi.org/10.1038/jhg.2010.103
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2010.103
Keywords
This article is cited by
-
Genome-wide association study identifies novel susceptibilities to adult moyamoya disease
Journal of Human Genetics (2023)
-
Moyamoya angiopathy in a case of Klinefelter syndrome
Child's Nervous System (2022)
-
The GC + CC genotype at position -418 in TIMP-2 promoter and the -1575GA/-1306CC genotype in MMP-2is genetic predisposing factors for prevalence of moyamoya disease
BMC Neurology (2014)
-
Recent Advances in Moyamoya Disease: Pathophysiology and Treatment
Current Neurology and Neuroscience Reports (2014)
-
Analysis of human leucocyte antigen genes in Caucasian patients with idiopathic Moyamoya angiopathy
Acta Neurochirurgica (2012)