Abstract
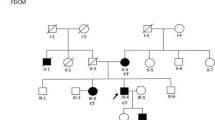
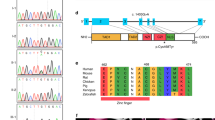
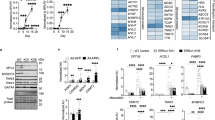
GATA6 is a member of the GATA family of transcription factors, and its expression and functions overlap with those of GATA4 during heart development. Mutations in GATA4 have been related to human congenital heart diseases (CHDs) in several studies, whereas mutations in GATA6 have only recently been reported in patients with persistent truncus arteriosus. Animal experiments have revealed critical roles for GATA6 in the development of the myocardium and cardiac morphogenesis, thereby highlighting the potential involvement of GATA6 defects in the pathogenesis of CHDs. Here, we screened the GATA6 in 270 individuals with sporadic CHDs by direct sequencing. After identification of the mutation, a luciferase reporter assay and real-time quantitative polymerase chain reaction were performed to detect functional changes in the mutant transcription factor. The same heterozygous missense mutation (Ser184Asn) was identified in three patients, including one with tetralogy of Fallot and two with atrial septal defects. This mutation was not found in 500 unrelated ethnically matched healthy subjects. Direct sequencing of this region in the parents of these three patients revealed the same mutation in one of the parents for each patient, and one of the parent carriers presented with a bicuspid aortic valve. Biological analysis revealed clearly decreased transcriptional activity of GATA6 Ser184Asn in vitro. All these data suggest that GATA6 Ser184Asn is a novel mutation associated with CHDs and has an important role in disease pathogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bruneau, B. G. The developmental genetics of congenital heart disease. Nature 451, 943–948 (2008).
Pierpont, M. E., Basson, C. T., Benson, D. W. Jr., Gelb, B. D., Giglia, T. M., Goldmuntz, E. et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115, 3015–3038 (2007).
Basson, C. T., Bachinsky, D. R., Lin, R. C., Levi, T., Elkins, J. A., Soults, J. et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat. Genet. 15, 30–35 (1997).
Garg, V., Kathiriya, I. S., Barnes, R., Schluterman, M. K., King, I. N., Butler, C. A. et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424, 443–447 (2003).
Li, Q. Y., Newbury-Ecob, R. A., Terrett, J. A., Wilson, D. I., Curtis, A. R., Yi, C. H. et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat. Genet. 15, 21–29 (1997).
Schott, J. J., Benson, D. W., Basson, C. T., Pease, W., Silberbach, G. M., Moak, J. P. et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science (New York, N.Y.) 281, 108–111 (1998).
Molkentin, J. D. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 275, 38949–38952 (2000).
Patient, R. K. & McGhee, J. D. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12, 416–422 (2002).
Peterkin, T., Gibson, A., Loose, M. & Patient, R. The roles of GATA-4, -5 and -6 in vertebrate heart development. Semin. Cell. Dev. Biol. 16, 83–94 (2005).
Hirayama-Yamada, K., Kamisago, M., Akimoto, K., Aotsuka, H., Nakamura, Y., Tomita, H. et al. Phenotypes with GATA4 or NKX2.5 mutations in familial atrial septal defect. Am. J. Med. Genet. A 135, 47–52 (2005).
Nemer, G., Fadlalah, F., Usta, J., Nemer, M., Dbaibo, G., Obeid, M. et al. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum. Mutat. 27, 293–294 (2006).
Okubo, A., Miyoshi, O., Baba, K., Takagi, M., Tsukamoto, K., Kinoshita, A. et al. A novel GATA4 mutation completely segregated with atrial septal defect in a large Japanese family. J. Med. Genet. 41, e97 (2004).
Posch, M. G., Perrot, A., Schmitt, K., Mittelhaus, S., Esenwein, E. M., Stiller, B. et al. Mutations in GATA4, NKX2.5, CRELD1, and BMP4 are infrequently found in patients with congenital cardiac septal defects. Am. J. Med. Genet. A 146A, 251–253 (2008).
Sarkozy, A., Conti, E., Neri, C., D’Agostino, R., Digilio, M. C., Esposito, G. et al. Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. J. Med. Genet. 42, e16 (2005).
Schluterman, M. K., Krysiak, A. E., Kathiriya, I. S., Abate, N., Chandalia, M., Srivastava, D. et al. Screening and biochemical analysis of GATA4 sequence variations identified in patients with congenital heart disease. Am. J. Med. Genet. A 143A, 817–823 (2007).
Tomita-Mitchell, A., Maslen, C. L., Morris, C. D., Garg, V. & Goldmuntz, E. GATA4 sequence variants in patients with congenital heart disease. J. Med. Genet. 44, 779–783 (2007).
Zhang, W., Li, X., Shen, A., Jiao, W., Guan, X. & Li, Z. GATA4 mutations in 486 Chinese patients with congenital heart disease. Eur. J. Med. Genet. 51, 527–535 (2008).
Lepore, J. J., Mericko, P. A., Cheng, L., Lu, M. M., Morrisey, E. E. & Parmacek, M. S. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J. Clin. Invest. 116, 929–939 (2006).
Maitra, M., Schluterman, M. K., Nichols, H. A., Richardson, J. A., Lo, C. W., Srivastava, D. et al. Interaction of Gata4 and Gata6 with Tbx5 is critical for normal cardiac development. Dev. Biol. 326, 368–377 (2009).
Xin, M., Davis, C. A., Molkentin, J. D., Lien, C. L., Duncan, S. A., Richardson, J. A. et al. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc. Natl Acad. Sci. USA 103, 11189–11194 (2006).
Zhao, R., Watt, A. J., Battle, M. A., Li, J., Bondow, B. J. & Duncan, S. A. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev. Biol. 317, 614–619 (2008).
Kodo, K., Nishizawa, T., Furutani, M., Arai, S., Yamamura, E., Joo, K. et al. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc. Natl Acad. Sci. USA 106, 13933–13938 (2009).
Li, H. H., Kedar, V., Zhang, C., McDonough, H., Arya, R., Wang, D. Z. et al. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J. Clin. Invest. 114, 1058–1071 (2004).
Fedak, P. W., Verma, S., David, T. E., Leask, R. L., Weisel, R. D. & Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 106, 900–904 (2002).
Nakamura, T., Colbert, M. C. & Robbins, J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ. Res. 98, 1547–1554 (2006).
Martin, L. J., Ramachandran, V., Cripe, L. H., Hinton, R. B., Andelfinger, G., Tabangin, M. et al. Evidence in favor of linkage to human chromosomal regions 18q, 5q and 13q for bicuspid aortic valve and associated cardiovascular malformations. Hum. Genet. 121, 275–284 (2007).
Morrisey, E. E., Ip, H. S., Tang, Z. & Parmacek, M. S. GATA-4 activates transcription via two novel domains that are conserved within the GATA-4/5/6 subfamily. J. Biol. Chem. 272, 8515–8524 (1997).
Charron, F., Paradis, P., Bronchain, O., Nemer, G. & Nemer, M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol. Cell. Biol. 19, 4355–4365 (1999).
Koutsourakis, M., Langeveld, A., Patient, R., Beddington, R. & Grosveld, F. The transcription factor GATA6 is essential for early extraembryonic development. Development (Cambridge, England) 126, 723–732 (1999).
Morrisey, E. E., Tang, Z., Sigrist, K., Lu, M. M., Jiang, F., Ip, H. S. et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes. Dev. 12, 3579–3590 (1998).
Srivastava, D. & Olson, E. N. A genetic blueprint for cardiac development. Nature 407, 221–226 (2000).
Acknowledgements
This work was supported by the National Science Fund of China (30425016, 30330290 and 30470961), the ‘973’ Program Fund of China (2007CB512100), the ‘863’ Program Fund of China (2007AA02Z438), the Program Fund for Outstanding Medical Academic Leaders of Shanghai, the Program Fund for Shanghai Subject Chief Scientists, the Yangtze Scholars Program Fund by the Ministry of Education of China, the Program Fund for Innovative Research Teams by the Ministry of Education of China (Y-HC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, X., Huo, Z., Liu, X. et al. A novel GATA6 mutation in patients with tetralogy of Fallot or atrial septal defect. J Hum Genet 55, 662–667 (2010). https://doi.org/10.1038/jhg.2010.84
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2010.84
Keywords
This article is cited by
-
Novel pathogenic GATA6 variant associated with congenital heart disease, diabetes mellitus and necrotizing enterocolitis
Pediatric Research (2024)
-
A novel causative functional mutation in GATA6 gene is responsible for familial dilated cardiomyopathy as supported by in silico functional analysis
Scientific Reports (2022)
-
Rare GATA6 variants associated with risk of congenital heart disease phenotypes in 200,000 UK Biobank exomes
Journal of Human Genetics (2022)
-
A gain-of-function ACTC1 3′UTR mutation that introduces a miR-139-5p target site may be associated with a dominant familial atrial septal defect
Scientific Reports (2016)