Abstract
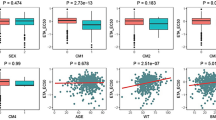
Warfarin is a commonly prescribed anticoagulant drug for the prevention of thromboembolic disorders. We investigated the contribution of genetic variations of four genes and clinical factors to warfarin dose requirement and provided a warfarin-dosing algorithm based on genetic and clinical variables in Korean patients. We recruited 564 Korean patients on stable anticoagulation. Single nucleotide polymorphisms (SNPs) for the VKORC1, CYP2C9, CYP4F2 and GGCX were analyzed. Using multiple regression analysis, we developed a model to predict the warfarin requirement. The SNPs of VKORC1, CYP2C9, CYP4F2 and GGCX showed significant correlation with warfarin dose. Patients with the 3730AA genotype received significantly higher doses of warfarin than those with the 3730GG (P=0.0001). For CYP2C9, the highest maintenance dose was observed in the patients with wild-type genotype compared with the variant allele carriers (P<0.0001). The multiple regression model including age, gender, body surface area (BSA), international normalized ratio (INR) and four genetic polymorphisms accounted for 35% of total variations in warfarin dose (R2=0.3499; P<0.0001). This study shows that age, gender, BSA, INR and VKORC1, CYP2C9 and CYP4F2 polymorphism affect warfarin dose requirements in Koreans. Translation of this knowledge into clinical guidelines for warfarin prescription may contribute to improve the efficacy and safety of warfarin treatment for Korean patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sconce, E. A., Khan, T. I., Wynne, H. A., Avery, P., Monkhouse, L., King, B. P. et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106, 2329–2333 (2005).
Wang, T. L., Li, H. L., Tjong, W. Y., Chen, Q. S., Wu, G. S., Zhu, H. T. et al. Genetic factors contribute to patient-specific warfarin dose for Han Chinese. Clin. Chim. Acta. 396, 76–79 (2008).
Pchelina, S. N., Sirotkina, O. V., Taraskina, A. E., Vavilova, T. V., Shwarzman, A. L. & Schwartz, E. I. The frequency of cytochrome P450 2C9 genetic variants in the Russian population and their associations with individual sensitivity to warfarin therapy. Thromb. Res. 115, 199–203 (2005).
Kimura, R., Miyashita, K., Kokubo, Y., Akaiwa, Y., Otsubo, R., Nagatsuka, K. et al. Genotypes of vitamin K epoxide reductase, gamma-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb. Res. 120, 181–186 (2007).
Miao, L., Yang, J., Huang, C. & Shen, Z. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur. J. Clin. Pharmacol. 63, 1135–1141 (2007).
Roper, N., Storer, B., Bona, R. & Fang, M. Validation and comparison of pharmacogenetics-based warfarin dosing algorithms for application of pharmacogenetic testing. J. Mol. Diagn. 12, 283–291 (2010).
Wen, M. S., Lee, M., Chen, J. J., Chuang, H. P., Lu, L. S., Chen, C. H. et al. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin. Pharmacol. Ther. 84, 83–89 (2008).
Limdi, N. A., Wadelius, M., Cavallari, L., Eriksson, N., Crawford, D. C., Lee, M. T. et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood 115, 3827–3834 (2010).
Villagra, D., Duconge, J., Windemuth, A., Cadilla, C. L., Kocherla, M., Gorowski, K. et al. CYP2C9 and VKORC1 genotypes in Puerto Ricans: a case for admixture-matching in clinical pharmacogenetic studies. Clin. Chim. Acta. 6, 1306–1311 (2010).
Sagreiya, H., Berube, C., Wen, A., Ramakrishnan, R., Mir, A., Hamilton, A. et al. Extending and evaluating a warfarin dosing algorithm that includes CYP4F2 and pooled rare variants of CYP29. Pharmacogenet. Genomics 20, 407–413 (2010).
Caldwell, M. D., Awad, T., Johnson, J. A., Gage, B. F., Falkowski, M., Gardina, P. et al. CYP4F2 genetic variant alters required warfarin dose. Blood 111, 4106–4112 (2008).
Patrinos, G. P. & Innocenti, F. Pharmacogenomics: paving the path to personalized medicine. Pharmacogenomics 11, 141–146 (2010).
Jonas, D. E. & McLeod, L Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol. Sci. 30, 375–386 (2009).
Langley, M. R., Booker, J. K., Evans, J. P., McLeod, H. L. & Weck, K. E. Validation of clinical testing for warfarin sensitivity: comparison of CYP2C9-VKORC1 genotyping assays and warfarin-dosing algorithms. J. Mol. Diagn. 11, 216–225 (2009).
D'Andrea, G., D'Ambrosio, R. L., Di Perna, P., Chetta, M., Santacroce, R., Brancaccio, V. et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 105, 645–649 (2005).
Lal, S., Jada, S. R., Xiang, X., Lim, W. T., Lee, E. J. & Chowbay, B. Pharmacogenetics of target genes across the warfarin pharmacological pathway. Clin. Pharmacokinet. 45, 1189–1200 (2006).
Yin, T. & Miyata, T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKORC1—rationale and perspectives. Thromb. Res. 120, 1–10 (2007).
Rieder, M. J., Reiner, A. P. & Rettie, A. E. γ-glutamyl carboxylase (GGCX) tagSNPs have limited utility for predicting warfarin maintenance dose. J. Thromb. Haemost. 5, 2227–2234 (2007).
Wadelius, M., Chen, L. Y., Downes, K., Ghori, J., Hunt, S., Eriksson, N. et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 5, 262–270 (2005).
Carlquist, J. F., Horne, B. D., Muhlestein, J. B., Lappé, D. L., Whiting, B. M., Kolek, M. J. et al. Genotypes of the cytochrome P450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J. Thromb. Thrombolysis 22, 191–197 (2006).
Ozer, N., Cam, N., Tangurek, B., Ozer, S., Uyarel, H., Oz, D. et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements in an adult Turkish population. Heart Vessels 25, 155–162 (2010).
Cho, H. J., Sohn, K. H., Park, H. M., Lee, K. H., Choi, B., Kim, S. et al. Factors affecting the interindividual variability of warfarin dose requirement in adult Korean patients. Pharmacogenomics 8, 329–337 (2007).
Takeuchi, F., Kashida, M., Okazaki, O., Tanaka, Y., Fukuda, S., Kashima, T. et al. Evaluation of pharmacogenetic algorithm for warfarin dose requirements in Japanese patients. Circ. J. 74, 977–982 (2010).
Bodin, L., Verstuyft, C., Tregouet, D. A., Robert, A., Dubert, L., Funck-Brentano, C. et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106, 135–140 (2005).
Acknowledgements
This study was supported by the Korea Food and Drug Administration (08172KFDA506) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, J., Kim, JO., Kang, D. et al. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J Hum Genet 56, 290–295 (2011). https://doi.org/10.1038/jhg.2011.4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2011.4
Keywords
This article is cited by
-
Evaluation of supervised machine learning algorithms in predicting the poor anticoagulation control and stable weekly doses of warfarin
International Journal of Clinical Pharmacy (2023)
-
Multiplex pyrosequencing method to determine CYP2C9*3, VKORC1*2, and CYP4F2*3 polymorphisms simultaneously: its application to a Korean population and comparisons with other ethnic groups
Molecular Biology Reports (2014)
-
Pharmacogenetics of warfarin: challenges and opportunities
Journal of Human Genetics (2013)
-
The prevalence of VKORC1 1639 G>A and CYP2C9*2*3 genotypes in patients that requiring anticoagulant therapy in Turkish population
Molecular Biology Reports (2012)
-
Impact of the CYP4F2 p.V433M Polymorphism on Coumarin Dose Requirement: Systematic Review and Meta-Analysis
Clinical Pharmacology & Therapeutics (2012)