Abstract
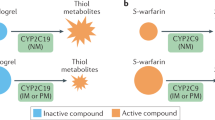
Pharmacogenomics, the study of the genomics of drug response and adverse effects, holds great promise for more effective individualized (personalized) medicine. Recent evidence supports a role of loss-of-function (LOF) variants in the cytochrome P450 enzyme CYP2C19 as a determinant of clopidogrel response. Patients given clopidogrel after percutaneous coronary intervention who carry LOF variants do not metabolize clopidogrel, a prodrug, into its active form resulting in decreased inhibition of platelet function and a higher likelihood of recurrent cardiovascular events. Despite a large body of evidence supporting clinical utility, adoption of anti-platelet pharmacogenetics into clinical practice has been slow. In this review, we summarize the pharmacokinetic, pharmacodynamic and clinical evidence, identify gaps in knowledge and other barriers that appear to be slowing adoption, and describe CYP2C19 pharmacogenetics implementation projects currently underway. Only when we surmount these barriers will clinicians be able to use pharmacogenetic information in conjunction with the history, physical examination and other medical tests and information to choose the most efficacious anti-platelet therapy for each individual patient.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kushner, F. G., Hand, M., Smith, S. C. Jr, King, S. B. 3rd, Anderson, J. L., Antman, E. M. et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update), a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 54, 2205–2241 (2009).
Anderson, J. L., Adams, C. D., Antman, E. M., Bridges, C. R., Califf, R. M., Casey, D. E. Jr et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 116, e148–e304 (2007).
Mega, J. L., Close, S. L., Wiviott, S. D., Shen, L., Walker, J. R., Simon, T. et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 376, 1312–1319 (2010).
Fisch, A. S., Perry, C. G., Stephens, S. H., Horenstein, R. B. & Shuldiner, A. R. Pharmacogenomics of anti-platelet and anti-coagulation therapy. Curr. Cardiol. Reports; in press (2013).
Ancrenaz, V., Daali, Y., Fontana, P., Besson, M., Samer, C., Dayer, P. et al. Impact of genetic polymorphisms and drug-drug interactions on clopidogrel and prasugrel response variability. Curr. Drug Metab. 11, 667–677 (2010).
Scott, S. A., Sangkuhl, K., Stein, C. M., Hulot, J.-S., Johnson, J. A., Roden, D. M. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy: 2013 Update. Clin. Pharmacol. Ther.; in press (2013).
Cattaneo, M. The platelet P2Y(1)(2) receptor for adenosine diphosphate: congenital and drug-induced defects. Blood 117, 2102–2112 (2011).
Gurbel, P. A., Bliden, K. P., Hiatt, B. L. & O'Connor, C. M. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107, 2908–2913 (2003).
Gurbel, P. A. & Tantry, U. S. Drug insight: clopidogrel nonresponsiveness. Nat. Clin. Pract. Cardiovasc. Med. 3, 387–395 (2006).
Angiolillo, D. J., Fernandez-Ortiz, A., Bernardo, E., Alfonso, F., Macaya, C., Bass, T. A. et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J. Am. Coll. Cardiol. 49, 1505–1516 (2007).
O'Donoghue, M. & Wiviott, S. D. Clopidogrel response variability and future therapies: clopidogrel: does one size fit all? Circulation 114, e600–e606 (2006).
Wang, T. H., Bhatt, D. L. & Topol, E. J. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur. Heart J. 27, 647–654 (2006).
Gilard, M., Arnaud, B., Le Gal, G., Abgrall, J. F. & Boschat, J. Influence of omeprazol on the antiplatelet action of clopidogrel associated to aspirin. J. Thromb. Haemost. 4, 2508–2509 (2006).
Lau, W. C., Gurbel, P. A., Watkins, P. B., Neer, C. J., Hopp, A. S., Carville, D. G. et al. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 109, 166–171 (2004).
Siller-Matula, J. M., Lang, I., Christ, G. & Jilma, B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J. Am. Coll. Cardiol. 52, 1557–1563 (2008).
Cayla, G., Hulot, J. S., O'Connor, S. A., Pathak, A., Scott, S. A., Gruel, Y. et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA 306, 1765–1774 (2011).
Gurbel, P. A., Bliden, K. P., Guyer, K., Cho, P. W., Zaman, K. A., Kreutz, R. P. et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J. Am. Coll. Cardiol. 46, 1820–1826 (2005).
Gurbel, P. A., Becker, R. C., Mann, K. G., Steinhubl, S. R. & Michelson, A. D. Platelet function monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 50, 1822–1834 (2007).
Angiolillo, D. J. & Alfonso, F. Platelet function testing and cardiovascular outcomes: steps forward in identifying the best predictive measure. Thromb. Haemost. 98, 707–709 (2007).
Bliden, K. P., DiChiara, J., Tantry, U. S., Bassi, A. K., Chaganti, S. K. & Gurbel, P. A. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J. Am. Coll. Cardiol. 49, 657–666 (2007).
Buonamici, P., Marcucci, R., Migliorini, A., Gensini, G. F., Santini, A., Paniccia, R. et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 49, 2312–2317 (2007).
Gurbel, P. A., Bliden, K. P., Zaman, K. A., Yoho, J. A., Hayes, K. M. & Tantry, U. S. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation 111, 1153–1159 (2005).
Gurbel, P. A., Antonino, M. J., Bliden, K. P., Dichiara, J., Suarez, T. A., Singla, A. et al. Platelet reactivity to adenosine diphosphate and long-term ischemic event occurrence following percutaneous coronary intervention: a potential antiplatelet therapeutic target. Platelets 19, 595–604 (2008).
Matetzky, S., Shenkman, B., Guetta, V., Shechter, M., Beinart, R., Goldenberg, I. et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 109, 3171–3175 (2004).
Dahlen, J. R., Price, M. J., Parise, H. & Gurbel, P. A. Evaluating the clinical usefulness of platelet function testing: considerations for the proper application and interpretation of performance measures. Thromb. Haemost. 109, 808–816 (2012).
Collet, J. P., Cuisset, T., Range, G., Cayla, G., Elhadad, S., Pouillot, C. et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N. Engl. J. Med. 367, 2100–2109 (2012).
Jneid, H. The 2012 ACCF/AHA focused update of the Unstable Angina/Non-ST-Elevation Myocardial Infarction (UA/NSTEMI) Guideline: a critical appraisal. Methodist. DeBakey Cardiovasc. J. 8, 26–30 (2012).
Scott, S. A., Sangkuhl, K., Shuldiner, A. R., Hulot, J. S., Thorn, C. F., Altman, R. B. et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet. Genomics 22, 159–165 (2012).
CYP2C19 allele nomenclature. http://www.cypalleles.ki.se/cyp2c19.htm/ (2013).
Scott, S. A., Sangkuhl, K., Gardner, E. E., Stein, C. M., Hulot, J. S., Johnson, J. A. et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 90, 328–332 (2011).
Scott, S. A., Sangkuhl, K., Gardner, E. E., Stein, C. M., Hulot, J. S., Johnson, J. A. et al Supplemental Table S1. Commonly Tested CYP2C19 Variant Alleles and their Effect on CYP2C19 Protein (ed. S1., ST) ((Clinical Pharmacology & Therapeutics, 2011).
Lewis, J., Stephens, S., Horenstein, R., O’Connell, J., Ryan, K., Peer, C. et al. The CYP2C19*17 variant is not independently associated with clopidogrel response. J. Thromb. Haemcost; in press (2013).
Hulot, J. S., Bura, A., Villard, E., Azizi, M., Remones, V., Goyenvalle, C. et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108, 2244–2247 (2006).
Umemura, K., Furuta, T. & Kondo, K. The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics in an active metabolite of clopidogrel in healthy subjects. J. Thromb. Haemost. 6, 1439–1441 (2008).
Gladding, P., Webster, M., Zeng, I., Farrell, H., Stewart, J., Ruygrok, P. et al. The pharmacogenetics and pharmacodynamics of clopidogrel response: an analysis from the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc. Interv. 1, 620–627 (2008).
Mega, J. L., Close, S. L., Wiviott, S. D., Shen, L., Hockett, R. D., Brandt, J. T. et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 360, 354–362 (2009).
Kim, K. A., Park, P. W., Hong, S. J. & Park, J. Y. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin. Pharmacol. Ther. 84, 236–242 (2008).
Fontana, P., Hulot, J. S., De Moerloose, P. & Gaussem, P. Influence of CYP2C19 and CYP3A4 gene polymorphisms on clopidogrel responsiveness in healthy subjects. J. Thromb. Haemost. 5, 2153–2155 (2007).
Brandt, J. T., Close, S. L., Iturria, S. J., Payne, C. D., Farid, N. A., Ernest, C. S. 2nd et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436 (2007).
Simon, T., Bhatt, D. L., Bergougnan, L., Farenc, C., Pearson, K., Perrin, L. et al. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin. Pharmacol. Ther. 90, 287–295 (2011).
Shuldiner, A. R., O'Connell, J. R., Bliden, K. P., Gandhi, A., Ryan, K., Horenstein, R. B. et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302, 849–857 (2009).
Simon, T., Verstuyft, C., Mary-Krause, M., Quteineh, L., Drouet, E., Meneveau, N. et al. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009).
Trenk, D., Hochholzer, W., Fromm, M. F., Chialda, L. E., Pahl, A., Valina, C. M. et al. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J. Am. Coll. Cardiol. 51, 1925–1934 (2008).
Collet, J. P., Hulot, J. S., Pena, A., Villard, E., Esteve, J. B., Silvain, J. et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373, 309–317 (2009).
Sibbing, D., Stegherr, J., Latz, W., Koch, W., Mehilli, J., Dorrler, K. et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur. Heart J. 30, 916–922 (2009).
Mega, J. L., Simon, T., Collet, J. P., Anderson, J. L., Antman, E. M., Bliden, K. et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304, 1821–1830 (2010).
FDA Announces New Boxed Warning on Plavix Alerts patients, health care professionals to potential for reduced effectiveness; Vol 2013 (2010).
Pare, G., Mehta, S. R., Yusuf, S., Anand, S. S., Connolly, S. J., Hirsh, J. et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N. Engl. J. Med. 363, 1704–1714 (2010).
Bhatt, D. L., Pare, G., Eikelboom, J. W., Simonsen, K. L., Emison, E. S., Fox, K. A. et al. The relationship between CYP2C19 polymorphisms and ischaemic and bleeding outcomes in stable outpatients: the CHARISMA genetics study. Eur. Heart J. 33, 2143–2150 (2012).
Holmes, M. V., Perel, P., Shah, T., Hingorani, A. D. & Casas, J. P. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA 306, 2704–2714 (2011).
Shuldiner, A. R., Vesely, M. R. & Fisch, A. CYP2C19 genotype and cardiovascular events. JAMA 307, 1482 (2012).
Mega, J. L., Topol, E. J. & Sabatine, M. S. CYP2C19 genotype and cardiovascular events. JAMA 307, 1482–1483 (2012).
Siasos, G., Tousoulis, D. & Stefanadis, C. CYP2C19 genotype and cardiovascular events. JAMA 307, 1483–1484 (2012).
Johnson, J. A., Roden, D. M., Lesko, L. J., Ashley, E., Klein, T. E. & Shuldiner, A. R. Clopidogrel: a case for indication-specific pharmacogenetics. Clin. Pharmacol. Ther. 91, 774–776 (2012).
Taubert, D., von Beckerath, N., Grimberg, G., Lazar, A., Jung, N., Goeser, T. et al. Impact of P-glycoprotein on clopidogrel absorption. Clin. Pharmacol. Ther. 80, 486–501 (2006).
Wang, X. D., Zhang, D. F., Liu, X. B., Lai, Y., Qi, W. G., Luo, Y. et al. Modified clopidogrel loading dose according to platelet reactivity monitoring in patients carrying ABCB1 variant alleles in patients with clopidogrel resistance. Eur. J. Intern. Med. 23, 48–53 (2012).
Su, J., Xu, J., Li, X., Zhang, H., Hu, J., Fang, R. et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis. PLoS One 7, e46366 (2012).
Zhu, H. J., Patrick, K. S., Yuan, H. J., Wang, J. S., Donovan, J. L., DeVane, C. L. et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am. J. Hum. Genet. 82, 1241–1248 (2008).
Lewis, J. P., Horenstein, R. B., Ryan, K., O’Connell, J. R., Gibson, Q., Mitchell, B. D. et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet. Genomics 23, 1–8 (2013).
Bouman, H. J., Schomig, E., van Werkum, J. W., Velder, J., Hackeng, C. M., Hirschhauser, C. et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 17, 110–116 (2011).
Price, M. J., Murray, S. S., Angiolillo, D. J., Lillie, E., Smith, E. N., Tisch, R. L. et al. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J. Am. Coll. Cardiol. 59, 1928–1937 (2012).
Lewis, J. P., Fisch, A. S., Ryan, K., O'Connell, J. R., Gibson, Q., Mitchell, B. D. et al. Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin. Pharmacol. Ther. 90, 568–574 (2011).
Hulot, J. S., Collet, J. P., Cayla, G., Silvain, J., Allanic, F., Bellemain-Appaix, A. et al. CYP2C19 but not PON1 genetic variants influence clopidogrel pharmacokinetics, pharmacodynamics, and clinical efficacy in post-myocardial infarction patients. Circ. Cardiovasc. Interv. 4, 422–428 (2011).
Kreutz, R. P., Nystrom, P., Kreutz, Y., Miao, J., Desta, Z., Breall, J. A. et al. Influence of paraoxonase-1 Q192R and cytochrome P450 2C19 polymorphisms on clopidogrel response. Clin. Pharmacol. 4, 13–20 (2012).
Simon, T., Steg, P. G., Becquemont, L., Verstuyft, C., Kotti, S., Schiele, F. et al. Effect of paraoxonase-1 polymorphism on clinical outcomes in patients treated with clopidogrel after an acute myocardial infarction. Clin. Pharmacol. Ther. 90, 561–567 (2011).
Lewis, J. P. & Shuldiner, A. R. Paraoxonase 1 Q192R variant and clopidogrel efficacy: fact or fiction? Circ. Cardiovasc. Genet. 5, 153–155 (2012).
Sibbing, D., Koch, W., Massberg, S., Byrne, R. A., Mehilli, J., Schulz, S. et al. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur. Heart J. 32, 1605–1613 (2011).
Szymezak, J., Moreau, C., Loriot, M. A., Durand, E., Van Viet, H., Desnos, M. et al. High on-clopidogrel platelet reactivity: genotyping can help to optimize antiplatelet treatment. Thromb. Res. 128, 92–95 (2011).
Staritz, P., Kurz, K., Stoll, M., Giannitsis, E., Katus, H. A. & Ivandic, B. T. Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int. J. Cardiol. 133, 341–345 (2009).
Wiviott, S. D., Braunwald, E., McCabe, C. H., Montalescot, G., Ruzyllo, W., Gottlieb, S. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Cannon, C. P., Harrington, R. A., James, S., Ardissino, D., Becker, R. C., Emanuelsson, H. et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 375, 283–293 (2010).
Roberts, J. D., Wells, G. A., Le May, M. R., Labinaz, M., Glover, C., Froeschl, M. et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 379, 1705–1711 (2012).
Price, M. J., Berger, P. B., Teirstein, P. S., Tanguay, J. F., Angiolillo, D. J., Spriggs, D. et al. Standard vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 305, 1097–1105 (2011).
Manolio, T. A., Chisholm, R. L., Ozenberger, B., Roden, D. M., Williams, M. S., Wilson, R. et al. Implementing genomic medicine in the clinic: the future is here. Genet. Med. 15, 258–267 (2013).
Beitelshees, A. L. Personalised antiplatelet treatment: a RAPIDly moving target. Lancet 379, 1680–1682 (2012).
Reese, E. S., Daniel Mullins, C., Beitelshees, A. L. & Onukwugha, E. Cost-effectiveness of cytochrome P450 2C19 genotype screening for selection of antiplatelet therapy with clopidogrel or prasugrel. Pharmacotherapy 32, 323–332 (2012).
Shuldiner, A. R., Relling, M. V., Peterson, J. F., Hicks, J. K., Freimuth, R. R., Sadee, W. et al. The pharmacogenomics research network translational pharmacogenetics program (TPP): overcoming challenges of real-world implementation. Clin. Pharmacol. Ther. (e-pub ahead of print 19 March 2013; doi:10.1038/clpt.2013.59).
Pulley, J. M., Denny, J. C., Peterson, J. F., Bernard, G. R., Vnencak-Jones, C. L., Ramirez, A. H. et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin. Pharmacol. Ther. 92, 87–95 (2012).
Acknowledgements
This work was supported by National Institutes of Health grants U01 GM074518 and U01 HL105198, the Baltimore Veterans Administration Geriatric Research and Education Clinical Center and the University of Maryland Medical Scientist Training Program (MSTP) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Shuldiner receives grant support from National Institutes of Health for the study of the pharmacogenomics of anti-platelet medications. He also serves as a consultant for United States Diagnostic Standards, Inc. Ms. Perry has no conflict of interest.
Rights and permissions
About this article
Cite this article
Perry, C., Shuldiner, A. Pharmacogenomics of anti-platelet therapy: how much evidence is enough for clinical implementation?. J Hum Genet 58, 339–345 (2013). https://doi.org/10.1038/jhg.2013.41
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2013.41
Keywords
This article is cited by
-
A synergic effect between CYP2C19*2, CYP2C19*3 loss-of-function and CYP2C19*17 gain-of-function alleles is associated with Clopidogrel resistance among Moroccan Acute Coronary Syndromes patients
BMC Research Notes (2018)
-
The value of using polymorphisms in anti-platelet therapy
Frontiers in Biology (2017)
-
Associations of CYP3A4, NR1I2, CYP2C19 and P2RY12 polymorphisms with clopidogrel resistance in Chinese patients with ischemic stroke
Acta Pharmacologica Sinica (2016)
-
Effect of genetic and coexisting polymorphisms on platelet response to clopidogrel in Chinese Han patients with acute coronary syndrome
Journal of Genetics (2016)
-
Clinical Pharmacokinetics and Pharmacodynamics of Clopidogrel
Clinical Pharmacokinetics (2015)