Abstract
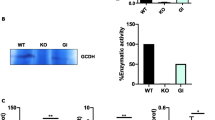
L-2-hydroxyglutaric aciduria (L2HGA) is an autosomal recessive neurometabolic disorder characterized essentially by the presence of elevated levels of L-2-hydroxyglutaric acid (LGA) in plasma, cerebrospinal fluid and urine. L2HGA is caused by a deficiency in the L2-Hydroxyglutaric dehydrogenase (L2HGDH) enzyme involved in the oxidation of LGA to the alpha 2-ketoglutarate. LGA has been proposed as an endo- and exogenous cytotoxic organic acid that induces free radical formation and generation of reactive oxygen species (ROS). In this report, we analyzed 14 L2HGA patients belonging to six unrelated consanguineous families the south of Tunisia. The patients were diagnosed with L2HGA disease confirmed on the presence of high level of LGA in urine. We analyzed the L2HGDH gene in all probands and identified the same c.241A>G homozygous mutation, which was previously reported in Tunisia. We also used intragenic single nucleotide length polymorphisms (SNPs) and two extragenic microsatellites flanking the L2HGDH gene to confirm the founder effect of c.241A>G mutation in the 14 studied cases. In addition, we carried out the measurement of the oxidative stress parameters in the plasma of L2HGA patients which revealed a significant increase in the malondialdehyde levels (MDA), a biomarker of lipid peroxydation, and the reduced glutathione (GSH). A diminution of the antioxidant enzyme activities including superoxide dismutase (SOD), glutathione peroxidase (GPx), was also observed.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Duran, M., Kamerling, J. P., Bakker, H. D., van Gennip, A. H. & Wadman, S. K. L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J.Inherit. Metab. Dis. 3, 109–112 (1980).
Chen, E., Nyhan, W. L., Jakobs, C., Greco, C. M., Barkovich, A. J., Cox, V. A. et al. L-2-Hydroxyglutaric aciduria: neuropathological correlations and first report of severe neurodegenerative disease and neonatal death. J. Inherit. Metab. Dis. 19, 335–343 (1996).
Barth, P. G., Wanders, R. J., Scholte, H. R., Abeling, N., Jakobs, C., Schutgens, R. B. et al. L-2-hydroxyglutaric aciduria and lactic acidosis. J. Inherit. Metab. Dis. 21, 251–254 (1998).
Hanefeld, F., Kruse, B., Bruhn, H. & Frahm, J. In vivo proton magnetic resonance spectroscopy of the brain in a patient with L-2-hydroxyglutaric acidemia. Pediatr. Res. 35, 614–616 (1994).
Latini, A., Scussiato, K., Rosa, R. B., Leipnitz, G., Llesuy, S., Belló-Klein, A. et al. Induction of oxidative stress by L-2-hydroxyglutaric acid in rat brain. J. Neurosci. Res. 74, 103–110 (2003).
Kölker, S., Ahlemeyer, B., Krieglstein, J. & Hoffmann, G. Contribution of reactive oxygen species to 3-Hydroxyglutarate neurotoxicity in primary neuronal cultures from chick embryo telencephalons. Pediatr. Res. 50, 76–82 (2001).
Yan, H. & Harding, J. J. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem. J. 328, 599–605 (1997).
Morgan, P. E., Dean, R. T. & Davies, M. J. Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch. Biochem. Biophys. 403, 259–269 (2002).
Topçu, M., Jobard, F., Halliez, S., Coskun, T., Yalçinkayal, C., Gerceker, F. O. et al. L-2-Hydroxyglutaric aciduria: identification of a mutant gene C14orf160, localized on chromosome 14q22.1. Hum. Mol. Genet. 3, 2803 (2004).
Rzem, R., Veiga-da-Cunha, M., Noël, G., Goffette, S., Nassogne, M. C., Tabarki, B. et al. A gene encoding a putative FAD-dependent L-2-hydroxyglutarate dehydrogenase is mutated in L-2-hydroxyglutaric aciduria. Proc. Natl Acad. Sci. USA 16849–16854 (2004).
Sass, J. O., Jobard, F., Topçu, M., Mahfoud, A., Werlé, E., Cure, S. et al. L-2-hydroxyglutaric aciduria: identification of ten novel mutations in the L2HGDH gene. J. Inherit. Metab. Dis. Suppl 2, S275–S279 (2008).
Vilarinho, L., Cardoso, M. L., Gaspar, P., Barbot, C., Azevedo, L., Diogo, L. et al. Novel L2HGDH mutations in 21 patients with L-2-hydroxyglutaric aciduria of Portuguese origin. Hum. Mutat. 26, 395–396 (2005).
Goffette, S. M., Duprez, T. P., Nassogne, M. C., Vincent, M. F., Jakobs, C. & Sindic, C. J. L-2-Hydroxyglutaric aciduria: clinical, genetic, and brain MRI characteristics in two adult sisters. Eur. J. Neurol. 13, 499–504 (2006).
Haliloglu, G., Jobard, F., Oguz, K. K., Anlar, B., Akalan, N., Coskun, T. et al. L-2-hydroxyglutaric aciduria and brain tumors in children with mutations in the L2HGDH gene: neuroimaging findings. Neuropediatrics 39, 119–122 (2008).
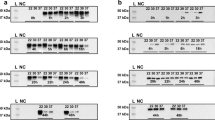
Larnaout, A., Amouri, R., Kefi, M. & Hentati, F. L-2-hydroxyglutaric aciduria: clinical and molecular study in three Tunisian families: identification of a new mutation and inter-familial phenotype variability. J. Inherit. Metab. Dis. 31 (Suppl 2), S375–S379 (2008).
Lewin, H. A. & Stewart-Haynes, J. A. A simple method for DNA extraction from leukocytes for use in PCR. Biotechniques 13, 522–524 (1992).
Bradford, M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Draper, H. H. & Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Method. Enzymol. 86, 421–431 (1990).
Flohe, L. & Gunzler, W. A. Assays of glutathione peroxidase. Method. Enzymol. 105, 114–121 (1984).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gel. Anal. Biochem. 44, 276–287 (1971).
Ellman, G. L. Sulphydryl groups. Arch. Biochem. Biophys. 82, 70–77 (1959).
Jollow, D. J., Mitchell, J. R., Zampaglione, N. & Gillete, J. R. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic intermediate. Pharmacology 11, 151–169 (1974).
Ben Arab, S., Masmoudi, S., Beltaief, N., Hachicha, S. & Ayadi, H. Consanguinity and endogamy in Northern Tunisia and its impact on non-syndromic deafness. Genet. Epidemiol. 27, 74–79 (2004).
Tlili, A., Ben Rebeh, I., Aifa-Hmani, M., Dhouib, H., Moalla, J., Tlili-Chouchène, J. et al. TMC1 but not TMC2 is responsible for autosomal recessive nonsyndromic hearing impairment in Tunisian families. Audiol. Neurootol. 13, 213–218 (2008).
Ben Saïd, M., Hmani-Aifa, M., Amar, I., Baig, S. M., Mustapha, M., Delmaghani, S. et al. High frequency of the p.R34X mutation in the TMC1 gene associated with nonsyndromic hearing loss is due to founder effects. Genet. Test. Mol. Biomarkers 14, 307–311 (2010).
Louhichi, N., Medhaffar, M., Hadjsalem, I., Mkaouar-Rebai, E., Fendri-Kriaa, N., Kanoun, H. et al. Congenital factor XIII deficiency caused by two mutations in eight Tunisian families: molecular confirmation of a founder effect. Ann. Hematol. 89, 499–504 (2010).
Chkioua, L., Khedhiri, S., Turkia, H. B., Tcheng, R., Froissart, R., Chahed, H. et al. Mucopolysaccharidosis type I: molecular characteristics of two novel alpha-L-iduronidase mutations in Tunisian patients. Diagn. Pathol. 6, 47 (2011).
Kammoun Jellouli, N., Hadj Salem, I., Ellouz, E., Ellouz, E., Louhichi, N., tlili, A. et al. Molecular confirmation of founder mutation c.-167A>G in Tunisian patients with PMLD disease. Gene 513, 233–238 (2013).
Halliwell, B. & Gutteridge, J. M. Free Radicals in Biology and Medicine, 3rd Edn (Oxford University Press: Oxford, 2001).
Halliwell, B. & Gutteridge, J. M. Lipid peroxidation in brain homogenates: the role of iron and hydroxyl radicals. J. Neurochem. 69, 1330–1331 (1997).
Deon, M., Sitta, A., Barschak, A. G., Coelho, D. M., Terroso, T., Schmitt, G. O. et al. Oxidative stress is induced in female carriers of X-linked adrenoleukodystrophy. Neurol. Sci. 266, 79–83 (2008).
Karthikeyan, V. J. & Lip, G. Y. Oxidative stress and hypertension. Int. J. Clin. Pract. 60, 1525–1527 (2006).
Fernández-Checa, J. C., Hirano, T., Tsukamoto, H. & Kaplowitz, N. Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol. 10, 469–475 (1993).
Loguercio, C., Blanco, F. D., De Girolamo, V., Disalvo, D., Nardi, G., Parente, A. et al. Ethanol consumption, amino acid and glutathione blood levels in patients with and without chronic liver disease. Alcohol. Clin. Exp. Res. 23, 1780–1784 (1999).
Acknowledgements
We are indebted to the families for their invaluable cooperation and for providing the blood samples. This research was funded by the Tunisian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Appendix The Tunisian Network on Mental Retardation study Marie Françoise Ben DRIDI, Néji TEBIB, Hatem Azouz, Hend Ben Khelifa, Amel Ben Chehida, Habiba Bouhamed Chaabouni, Ridha M’rad, Myriam Chaabouni, Lamia Ben Jemaa, Faouzi Maaloul, Naziha Kaabachi, Haifa Sanhaji, Fahmi Nasrallah, Ali Saad Hatem Elghezal, Moez Gribaa, Soumaya Mougou, Ines Ben Abdallah, Ramzi Zemni, Foued Haj Salama, Elyes Chabchoub, Amani Achour, Ahmed Sahloul Essoussi, Lamia Boughamoura, Najla Souayah, Jihene Bouguila, Abdelhedi Miled, Salima Ferchichi, Henda Chahed, Chahnez Charfi Triki, Houda Ben Othman, Emna Ellouz, Fatma Kamoun, Faiza Fakhfakh, Hassen Kamoun, Neila Belghuith, Nourhene Fendri, Nadege Kamoun, Fatma Makni Ayedi, Kamel Jamoussi, Mouna Turki, Meriam Messeddi.
Rights and permissions
About this article
Cite this article
Jellouli, N., Hadj Salem, I., Ellouz, E. et al. Founder effect confirmation of c.241A>G mutation in the L2HGDH gene and characterization of oxidative stress parameters in six Tunisian families with L-2-hydroxyglutaric aciduria. J Hum Genet 59, 216–222 (2014). https://doi.org/10.1038/jhg.2014.4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2014.4
Keywords
This article is cited by
-
Loss of function variants in L2HGDH gene causing l-2-hydroxyglutaric aciduria
Acta Neurologica Belgica (2023)
-
l-2-Hydroxyglutaric Acid Administration to Neonatal Rats Elicits Marked Neurochemical Alterations and Long-Term Neurobehavioral Disabilities Mediated by Oxidative Stress
Neurotoxicity Research (2023)
-
A novel protein truncating mutation in L2HGDH causes L-2-hydroxyglutaric aciduria in a consanguineous Pakistani family
Metabolic Brain Disease (2022)
-
Oxidative stress among L-2-hydroxyglutaric aciduria disease patients: evaluation of dynamic thiol/disulfide homeostasis
Metabolic Brain Disease (2019)
-
Identification of novel L2HGDH mutation in a large consanguineous Pakistani family- a case report
BMC Medical Genetics (2018)