Abstract
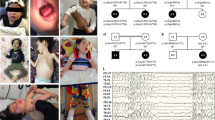
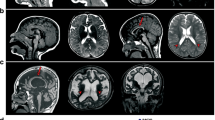
Recent progress in genetic analysis reveals that a significant proportion of cryptogenic epileptic encephalopathies are single-gene disorders. Mutations in numerous genes for early-onset epileptic encephalopathies have been rapidly identified, including in SPTAN1, which encodes α-II spectrin. The aim of this review is to delineate SPTAN1 encephalopathy as a distinct clinical syndrome. To date, a total of seven epileptic patients with four different in-frame SPTAN1 mutations have been identified. The major clinical features of SPTAN1 mutations include epileptic encephalopathy with hypsarrhythmia, no visual attention, acquired microcephaly, spastic quadriplegia and severe intellectual disability. Brainstem and cerebellar atrophy and cerebral hypomyelination, as observed by magnetic resonance imaging, are specific hallmarks of this condition. A milder variant is characterized by generalized epilepsy with pontocerebellar atrophy. Only in-frame SPTAN1 mutations in the last two spectrin repeats in the C-terminal region lead to dominant negative effects and these specific phenotypes. The last two spectrin repeats are required for α/β spectrin heterodimer associations and the mutations can alter heterodimer formation between the two spectrins. From these data we suggest that SPTAN1 encephalopathy is a distinct clinical syndrome owing to specific SPTAN1 mutations. It is important that this syndrome is recognized by pediatric neurologists to enable proper diagnostic work-up for patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Berg, A. T ., Berkovic, S. F ., Brodie, M. J ., Buchhalter, J ., Cross, J. H . & van Emde Boas, W . et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51, 676–685 (2010).
Sharma, S . & Prasad, A. N. Genetic testing of epileptic encephalopathies of infancy: an approach. Can. J. Neurol. Sci. 40, 10–16 (2013).
Dulac, O. Epileptic encephalopathy. Epilepsia 42, 23–26 (2001).
Strømme, P ., Mangelsdorf, M. E ., Scheffer, I. E . & Gécz, J. Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 24, 266–268 (2002).
Kato, M ., Das, S ., Petras, K ., Sawaishi, Y . & Dobyns, W. B. Polyalanine expansion of ARX associated with cryptogenic West syndrome. Neurology 61, 267–276 (2003).
Kalscheuer, V. M ., Tao, J ., Donnelly, A ., Hollway, G ., Schwinger, E . & Kübart, S . et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am. J. Hum. Genet. 72, 1401–1411 (2003).
Kato, M ., Saitoh, S ., Kamei, A ., Shiraishi, H ., Ueda, Y . & Akasaka, M . et al. A longer polyalanine expansion mutation in the ARX gene causes early infantile epileptic encephalopathy with suppression-burst pattern (Ohtahara syndrome). Am. J. Hum. Genet. 81, 361–366 (2007).
Saitsu, H ., Kato, M ., Mizuguchi, T ., Hamada, K ., Osaka, H . & Tohyama, J . et al. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat. Genet. 40, 782–788 (2008).
Molinari, F ., Kaminska, A ., Fiermonte, G ., Boddaert, N ., Raas-Rothschild, A . & Plouin, P . et al. Mutations in the mitochondrial glutamate carrier SLC25A22 in neonatal epileptic encephalopathy with suppression bursts. Clin. Genet. 76, 188–194 (2009).
Paciorkowski, A. R ., Thio, L. L . & Dobyns, W. B. Genetic and biologic classification of infantile spasms. Pediatr. Neurol. 45, 355–367 (2011).
Nakamura, K ., Kato, M ., Osaka, H ., Yamashita, S ., Nakagawa, E . & Haginoya, K . et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology 81, 992–998 (2013).
Nakamura, K ., Kodera, H ., Akita, T ., Shiina, M ., Kato, M . & Hoshino, H . et al. De Novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 93, 496–505 (2013).
Kodera, H ., Nakamura, K ., Osaka, H ., Maegaki, Y ., Haginoya, K . & Mizumoto, S . et al. De novo mutations in SLC35A2 encoding a UPD-galactose transporter cause early-onset epileptic encephalopathy. Hum. Mutat. 34, 1708–1714 (2013).
Ng, B. G ., Buckingham, K. J ., Raymond, K ., Kircher, M ., Turner, E. H . & He, M . et al. Mosaicism of the UDP-galactose transporter SLC35A2 causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 92, 632–636 (2013).
Vissers, L. E. L. M ., de Ligt, J ., Gillissen, C ., Janssen, I ., Steehouwer, M . & de Vries, P . et al. A de novo paradigm for mental retardation. Nat. Genet. 42, 1109–1112 (2010).
Kamien, B. A ., Cardamone, M ., Lawson, J. A . & Sachdev, R. A genetic diagnostic approach to infantile epileptic encephalopathies. J. Clin. Neurosci. 19, 934–941 (2012).
Mastrangelo, M . & Leuzzi, V. Genes of early-onset epileptic encephalopathies: from genotype to phenotype. Pediatr. Neurol. 46, 24–31 (2012).
Saitsu, H ., Tohyama, J ., Kumada, T ., Egawa, K ., Hamada, K . & Okada, I . et al. Dominant-negative mutations in α-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadripkegia, and developmental delay. Am. J. Hum. Genet. 86, 881–891 (2010).
Voas, M. G ., Lyons, D. A ., Naylor, S. G ., Arana, N ., Rasband, M. N . & Talbot, W. S. αII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr. Biol. 17, 562–568 (2007).
Tohyama, J ., Akasaka, N ., Osaka, H ., Maegaki, Y ., Kato, M . & Saito, N . et al. Early onset West syndrome with cerebral hypomyelination and reduced cerebral white matter. Brain Dev. 30, 349–355 (2008).
Writzl, K ., Primec, Z. R ., Strazisar, B. G ., Osredkar, D ., Pecaric-Meglic, N . & Kranjc, B. S . et al. Early onset West syndrome with severe hypomyelination and coloboma-like optic discs in a girl with SPTAN1 mutation. Epilepsia 53, e106–e110 (2012).
Nonoda, Y ., Saito, Y ., Nagai, S ., Sasaki, M ., Iwasaki, T . & Matsumoto, N . et al. Progressive diffuse brain atrophy in West syndrome with marked hypomyalination due to SPTAN1 gene mutation. Brain Dev. 35, 280–283 (2013).
Hamdan, F. F ., Saitsu, H ., Nishiyama, K ., Gauthier, J ., Dobrzeniecka, S . & Spingelman, D . et al. Identification of a novel in-frame de novo mutation in SPTAN1 in intellectual disability and pontocerebellar atrophy. Eur. J. Hum. Genet. 20, 796–800 (2012).
Lemke, J. R ., Riesch, E ., Scheurenbrand, T ., Schubach, M ., Wilhelm, C . & Steiner, I . et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 53, 1378–1398 (2012).
Allen, A. S ., Berkovic, S.F ., Cossette, P ., Delanty, N ., Dlugos, D . & Eichler, E. E . et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
Veeramah, K. R ., Johnstone, L ., Karafet, T. M ., Wolf, D ., Sprissler, R . & Salogiannis, J . et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia 54, 1270–1281 (2013).
Martin, H. C ., Kim, G. E ., Pagnamenta, A. T ., Murakami, Y ., Carvill, G. L . & Meyer., E . et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum. Mol. Genet. 23, 3200–3211 (2014).
de Ligt, J ., Willemsen, M. H ., van Bon, B. W. M ., Kleefstra, T ., Yntema, H. G . & Kroes, T . et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921–1929 (2012).
Bennett, V . & Baines, A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81, 1353–1392 (2001).
Bennett, V . & Healy, J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol. Med. 14, 28–36 (2008).
Machnicka, B ., Czogalla, A ., Hryniewicz-Jankowska, A ., Bogusławska, D. M ., Grochowalska, R . & Heger, E . et al. Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim. Biophys. Acta 1838, 620–634 (2014).
Ikeda, Y ., Dick, K. A ., Weatherspoon, M. R ., Gincel, D ., Armbrust, K. R . & Dalton, J. C . et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 38, 184–190 (2006).
Perrotta, S ., Gallagher, P. G . & Mohandas, N. Hereditary spherocytosis. Lancet 372, 1411–1426 (2008).
Meary, F ., Metral, S ., Ferreira, C ., Eladari, D ., Colin, Y . & Lecomte, M. C . et al. A mutant αII-spectrin designed to resist calpain and caspase cleavage questions the functional importance of this process in vivo. J. Biol. Chem. 282, 14226–14237 (2007).
Mignot, C ., Moutard, M. L ., Trouillard, O ., Gourfinkel-An, I ., Jacquette, A . & Arveiler, B . et al. STXBP1-related encephalopathy presenting as infantile spasms and generalized tremor in three patients. Epilepsia 52, 1820–1827 (2011).
Campbell, I. M ., Yatsenko, S. A ., Hixson, P ., Reimschisel, T ., Thomas, M . & Wilson, W . et al. Novel 9q34.11 gene deletions encompassing combinations of four Mendelian disease genes: STXBP1 SPTAN1 ENG, and TOR1A. Genet. Med. 14, 868–876 (2012).
Barcia, G ., Chemaly, N ., Gobin, S ., Milh, M ., Van Bogaert, P . & Barnerias, C . et al. Early epileptic encephalopathies associated with STXBP1 mutations: could we better delineate the phenotype? Eur. J. Med. Genet. 57, 15–20 (2014).
Saitsu, H ., Kato, M ., Shimono, M ., Senju, A ., Tanabe, S . & Kimura, T . et al. Association of genomic deletions in the STXBP1 gene with Ohtahara syndrome. Clin. Genet. 81, 399–402 (2012).
Mastrangelo, M ., Peron, A ., Spaccini, L ., Novara, F ., Scelsa, B . & Introvini, P . et al. Neonatal suppression-burst without epileptic sezures: expanding the electroclinical phenotype of STXBP1-related, early-onset encephalopathy. Epileptic Disord. 15, 55–61 (2013).
Tzschach, A ., Grasshoff, U ., Schäferhoff, K ., Bonin, M ., Dufke, A . & Wolff, M . et al. Interstitial 9q34.11-q34.13 deletion in a patient with severe intellectual disability, hydrocephalus, and cleft lip/palate. Am. J. Med. Genet. A 158A, 1709–1712 (2012).
Hamdan, F. F ., Piton, A ., Gauthier, J ., Lortie, A ., Dubeau, F . & Dobrzeniecka, S . et al. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Ann. Neurol. 65, 748–753 (2009).
Hamdan, F.F ., Gauthier, J ., Dobrzeniecka, S ., Lortie, A ., Mottron, L . & Vanasse, M . et al. Intellectual disability without epilepsy associated with STXBP1 disruption. Eur. J. Hum. Genet. 19, 607–609 (2011).
Saitsu, H ., Kato, M ., Okada, I ., Orii, K.E ., Higuchi, T . & Hoshino, H . et al. STXBP1 mutations in early infantile epileptic encephalopathy with suppression-burst pattern. Epilepsia 51, 2397–2405 (2010).
Salonen, R ., Somer, M ., Haltia, M ., Lorentz, M . & Norio, R. Progressive encephalopathy with edema, hypsarrhythmia, and optic atrophy (PEHO syndrome). Clin. Genet. 39, 287–293 (1991).
Rankin, J ., Brown, R ., Dobyns, W.B ., Harington, J ., Patel, J . & Quinn, M . et al. Pontocerebellar hypoplasia type 6: a British case with PEHO-like features. Am. J. Med. Genet. A 152A, 2079–2084 (2010).
Saitsu, H ., Kato, M ., Osaka, H ., Moriyama, N ., Horita, H . & Nishiyama, K . et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia 53, 1441–1449 (2012).
Najm, J ., Horn, D ., Wimplinger, I ., Golden, J. A ., Chizhikov, V. V . & Sudi, J . et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat. Genet. 40, 1065–1067 (2008).
Deprez, L ., Weckhuysen, S ., Holmgren, P ., Suls, A ., Van Dyck, T . & Goossens, D . et al. Clinical spectrum of early-onset epileptic encephalopaties associated with STXBP1 mutations. Neurology 75, 1159–1165 (2010).
Jaeken, J ., Detheux, M ., Van Maldergem, L ., Foulon, M ., Carchon, H . & Van Schaftingen, E. 3-Phosphoglycerate dehydrogenase deficiency: an inborn error of serine biosynthesis. Arch. Dis. Child 74, 542–545 (1996).
Vijayakumar, K ., Gunny, R ., Grunewald, S ., Carr, L ., Chong, K. W . & DeVile, C . et al. Clinical neuroimaging features and outcome in molybdenum cofactor deficiency. Pediatr. Neurol. 45, 246–252 (2011).
Kanazawa, K ., Kumada, S ., Kato, M ., Saitsu, H ., Kurihara, E . & Matsumoto, N. Choreo-ballistic movement in a case carrying a missense mutation in syntaxin binding protein 1 gene. Mov. Disord. 25, 2265–2267 (2010).
Guerrini, R ., Moro, F ., Kato, M ., Barkovich, A. J ., Shiihara, T . & McShane, M. A . et al. Expansion of the first PolyA tract of ARX causes infantile spasms and status dystonicus. Neurology 69, 427–433 (2007).
Guerrini, R . & Parrini, E. Epilepsy in Rett syndrome, and CDKL5- and FOXG1-gene-related encephalopathies. Epilepsia 53, 2067–2078 (2012).
Acknowledgements
We thank the patients and their parents for their valuable contributions. We are grateful to Dr Masumi Inagaki for critical advice; Dr Masayuki Sasaki for his continuous support; and Dr Noriyuki Akasaka, Dr Yutaka Nonoda, Dr Barbara G.Stražišar and Dr Karin Writzl for providing patients’ information. This work was supported by the Ministry of Health, Labour and Welfare of Japan; the Japan Society for the Promotion of Science (a Grant-in-Aid for Scientific Research (B), and a Grant-in-Aid for Scientific Research (A)); the Takeda Science Foundation; the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems; the Strategic Research Program for Brain Sciences; and a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription cycle) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tohyama, J., Nakashima, M., Nabatame, S. et al. SPTAN1 encephalopathy: distinct phenotypes and genotypes. J Hum Genet 60, 167–173 (2015). https://doi.org/10.1038/jhg.2015.5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2015.5
This article is cited by
-
Impaired OTUD7A-dependent Ankyrin regulation mediates neuronal dysfunction in mouse and human models of the 15q13.3 microdeletion syndrome
Molecular Psychiatry (2023)
-
SPTAN1 variants likely cause autosomal recessive complicated hereditary spastic paraplegia
Journal of Human Genetics (2022)
-
Identification of potential chemical compounds enhancing generation of enucleated cells from immortalized human erythroid cell lines
Communications Biology (2021)
-
EEG Monitoring of the Epileptic Newborn
Current Neurology and Neuroscience Reports (2020)
-
A customized high-resolution array-comparative genomic hybridization to explore copy number variations in Parkinson’s disease
neurogenetics (2016)