Abstract
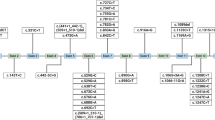
Phenylketonuria (PKU), the most common inborn error of amino acid metabolism, is caused by mutations in the phenylalanine-4-hydroxylase (PAH) gene. This study aimed to assess the genotype–phenotype correlation in the PKU Spanish population and the usefulness in establishing genotype-based predictions of BH4 responsiveness in our population. It involved the molecular characterization of 411 Spanish PKU patients: mild hyperphenylalaninemia non-treated (mild HPA-NT) (34%), mild HPA (8.8%), mild-moderate (20.7%) and classic (36.5%) PKU. BH4 responsiveness was evaluated using a 6R-BH4 loading test. We assessed genotype–phenotype associations and genotype–BH4 responsiveness in our population according to literature and classification of the mutations. The mutational spectrum analysis showed 116 distinct mutations, most missense (70.7%) and located in the catalytic domain (62.9%). The most prevalent mutations were c.1066-11G>A (9.7%), p.Val388Met (6.6%) and p.Arg261Gln (6.3%). Three novel mutations (c.61-13del9, p.Ile283Val and p.Gly148Val) were reported. Although good genotype–phenotype correlation was observed, there was no exact correlation for some genotypes. Among the patients monitored for the 6R-BH4 loading test: 102 were responders (87, carried either one or two BH4-responsive alleles) and 194 non-responders (50, had two non-responsive mutations). More discrepancies were observed in non-responders. Our data reveal a great genetic heterogeneity in our population. Genotype is quite a good predictor of phenotype and BH4 responsiveness, which is relevant for patient management, treatment and follow-up.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Walter, J. H., Lachmann, R. H., Burgard, P. in Inborn Metabolic Diseases. Diagnosis and Treatment 5th edn (eds Saudubray, J.-M., van den Berghe, G. & Walter, J. H.) 251–264 (Springer-Verlag, Berlin Heidelberg, Germany, 2012).
Donlon, J ., Sarkissian, C ., Levy, H . & Scriver, C. R . in The Online Metabolic and Molecular Bases of Inherited Disease (eds Valle, D., Beaudet, A. L., Vogelstein, B., Kinzler, K. W., Antonarakis, S. E., Ballabio, A. et al.) (McGraw-Hill, New York, NY, USA, 2014).
Camp, K. M., Parisi, M. A., Acosta, P. B., Berry, G. T., Bilder, D. A., Blau, N. et al. Phenylketonuria Scientific Review Conference: state of the science and future research needs. Mol. Genet. Metab. 112, 87–122 (2014).
Gassió, R., Artuch, R., Vilaseca, M. A., Fusté, E., Boix, C., Sans, A. et al. Cognitive functions in classic phenylketonuria and mild hyperphenylalaninaemia: experience in a paediatric population. Dev. Med. Child. Neurol. 47, 443–448 (2005).
Viau, K. S., Wengreen, H. J., Ernst, S. L., Cantor, N. L., Furtado, L. V. & Longo, N. Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J. Inherit. Metab. Dis. 34, 963–971 (2011).
Blau, N., van Spronsen, F. J. & Levy, H. L. Phenylketonuria. Lancet 376, 1417–1427 (2010).
Centerwall, W. R., Centerwall, S. A., Armon, V. & Mann, L.B. Phenylketonuria. II. Results of treatment of infants and young children. A report of 10 cases. J. Pediatr. 59, 102–118 (1961).
Fisch, R. O., Gravem, H. J. & Feinberg, S. B. Growth and bone characteristics of phenylketonurics. Comparative analysis of treated and untreated phenylketonuric children. Am. J. Dis. Child. 112, 3–10 (1996).
Sutherland, B. S., Umbarger, B. & Berry, H. K. The treatment of phenylketonuria: a decade of results. Am. J. Dis. Child. 111, 505 (1966).
Strisciuglio, P. & Concolino, D. New strategies for the treatment of phenylketonuria (PKU). Metabolites 4, 1007–1017 (2014).
Ney, D. M., Blank, R. D. & Hansen, K. E. Advances in the nutritional and pharmacological management of phenylketonuria. Curr. Opin. Clin. Nutr. Metab. Care 17, 61–68 (2014).
Kure, S., Hou, D. C., Ohura, T., Iwamoto, H., Suzuki, S., Sugiyama, N. et al. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J. Pediatr. 135, 375–378 (1999).
Douglas, T. D., Ramakrishnan, U., Kable, J. A. & Singh, R. H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Health Qual. Life Outcomes 11, 218 (2013).
Burton, B. K., Bausell, H., Katz, R., Laduca, H. & Sullivan, C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU). Mol. Genet. Metab. 101, 110–114 (2010).
Humphrey, M., Nation, J., Francis, I. & Boneh, A. Effect of tetrahydrobiopterin on Phe/Tyr ratios and variation in Phe levels in tetrahydrobiopterin responsive PKU patients. Mol. Genet. Metab. 104, 89–92 (2011).
Leuret, O., Barth, M., Kuster, A., Eyer, D., de Parscau, L., Odent, S. et al. Efficacy and safety of BH4 before the age of 4 years in patients with mild phenylketonuria. J. Inherit. Metab. Dis. 35, 975–981 (2012).
Michals-Matalon, K. Sapropterin dihydrochloride, 6-R-L-erythro-5,6,7,8-tetrahydrobiopterin, in the treatment of phenylketonuria. Expert Opin. Investig. Drugs 17, 245–251 (2008).
Lidsky, A. S., Robson, K. J. H., Thirumalachary, C., Barker, P. E., Ruddle, F. H. & Woo, S. L. C. The PKU locus in man is on chromosome 12. Am. J. Hum. Genet. 36, 527–533 (1984).
Woo, S. L. C., Lidsky, A., Law, M. & Kao, F. T. Regional mapping of the human phenylalanine hydroxylase gene and PKU locus to 12q21-qter (Abstract). Am. J. Hum. Genet. 36, 210S (1984).
Waters, P. J., Parniak, M. A., Nowacki, P. & Scriver, C. R. In vitro expression analysis of mutations in phenylalanine hydroxilase: linking genotype to phenotype and structure to function. Hum. Mutat. 11, 4–17 (1998).
Quirk, M. E., Dobrowolski, S. F., Nelson, B. E., Coffee, B. & Singh, R. H. Utility of phenylalanine hydroxylase genotype for tetrahydrobiopterin responsiveness classification in patients with phenylketonuria. Mol. Genet. Metab. 107, 31–36 (2012).
Blau, N., Hennermann, J.B., Langenbeck, U. & Lichter-Konecki, U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol. Genet. Metab. 104, S2–S9 (2011).
Zhu, T., Ye, J., Han, L., Qiu, W., Zhang, H., Liang, L. et al. Variations in genotype-phenotype correlations in phenylalanine hydroxylase deficiency in Chinese Han population. Gene 529, 80–87 (2013).
Polak, E., Ficek, A., Radvanszky, J., Soltysova, A., Urge, O., Cmelova, E. et al. Phenylalanine hydroxylase deficiency in the Slovak population: genotype-phenotype correlations and genotype-based predictions of BH4-responsiveness. Gene 526, 347–355 (2013).
Bueno, M. A., González-Lamuño, D., Delgado-Pecellín, C., Aldámiz-Echevarría, L., Pérez, B., Desviat, L.R. et al. Molecular epidemiology and genotype-phenotype correlation in phenylketonuria patients from South Spain. J. Hum. Genet. 58, 279–284 (2013).
Martínez Pardo, M., Bélanger-Quintana, A., García Muñoz, M. J., Desviat, L., Pérez, B. & Ugarte, M. Protocolo de diagnóstico, tratamiento y seguimiento de las hiperfenilalaninemias. http://www.ae3com.eu/protocolos/protocolo4.pdf.
Asociación Española para el Estudio de los Errores Congénitos del Metabolismo (AECOM) Guía Clínica para el diagnostic, tratamiento y registro de pacientes con hiperfenilalaninemia en España, (R.B. Servicios Editoriales, S. L., Spain, 2011).
Aldámiz-Echevarría, L., Bueno, M. A., Couce, M. L., Lage, S., Dalmau, J., Vitoria, I. et al. Tetrahydrobiopterin therapy vs phenylalanine-restricted diet: impact on growth in PKU. Mol. Genet. Metab. 109, 331–338 (2013).
Bueno, M. A., Lage, S., Delgado, C., Andrade, F., Couce, M. L., González-Lamuño, D. et al. New evidence for assessing tetrahydrobiopterin (BH(4)) responsiveness. Metabolism 61, 1809–1816 (2012).
Pey, A. L., Stricher, F., Serrano, L. & Martinez, A. Predicted effects of missense mutations on native-state stability account for phenotypic outcome in phenylketonuria, a paradigm of misfolding diseases. Am. J. Hum. Genet. 81, 1006–1024 (2007).
Zurflüh, M. R., Zschocke, J., Lindner, M., Feillet, F., Chery, C., Burlina, A. et al. Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum. Mutat. 29, 167–175 (2008).
Blau N., Yue W., Perez B. BIOPKUdb: database of patients and genotypes causing HPA/PKU incl. BH4-responsive phenotype. BIOPKU database; http://www.biopku.org/biopku/ (programmed in Access 2003, Microsoft, Redmond, WA; accessed 15 September 2015).
Khemir, S., Tebib, N., Nasrallah, F., Ben Nour, F., Mizouni, H., Elasmi, M. et al. Phenylketonuria in Tunisian institutions for the mentally handicapped. Arch. Dis. Child. 94, 647–648 (2009).
Rivera, I., Mendes, D., Afonso, A., Barroso, M., Ramos, R., Janeiro, P. et al. Phenylalanine hydroxylase deficiency: molecular epidemiology and predictable BH4-responsiveness in South Portugal PKU patients. Mol. Genet. Metab. 104, S86–S92 (2011).
Zschocke, J. Phenylketonuria mutations in Europe. Hum. Mutat. 21, 345–356 (2003).
Kim, N. K., Kim, A. R., Park, K. T., Kim, S. Y., Kim, M. Y., Nam, J. Y. et al. Whole-exome sequencing reveals diverse modes of inheritance in sporadic mild to moderate sensorineural hearing loss in a pediatric population. Genet. Med. 17, 901–911 (2015).
Bach, J. E., Wolf, B., Oldenburg, J., Müller, C. R. & Rost, S. Identification of deep intronic variants in 15 haemophilia A patients by next generation sequencing of the whole factor VIII gene. Thromb. Haemost. 114, 757–767 (2015).
Santos, L. L., Fonseca, C. G., Starling, A. L., Januario, J. N., Aguiar, M. J., Peixoto, M.G. et al. Variations in genotype-phenotype correlations in phenylketonuria patients. Genet. Mol. Res. 9, 1–8 (2010).
Guldberg, P., Rey, F., Zschocke, J., Romano, V., Francois, B., Michiels, L. et al. A European multicenter study of phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am. J. Hum. Genet. 63, 71–79 (1998).
Blau, N., Shen, N. & Carducci, C. Molecular genetics and diagnosis of phenylketonuria: state of the art. Expert Rev. Mol. Diagn. 14, 655–671 (2014).
Pey, A. L., Desviat, L. R., Gámez, A., Ugarte, M. & Pérez, B. Phenylketonuria: genotype-phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum. Mutat. 21, 370–378 (2003).
Dipple, K. M. & McCabe, E. R. Modifier genes convert ’simple‘ Mendelian disorders to complex traits. Mol. Genet. Metab. 71, 43–50 (2000).
Heintz, C., Cotton, R. G. & Blau, N. Tetrahydrobiopterin, its mode of action on phenylalanine hydroxylase, and importance of genotypes for pharmacological therapy of phenylketonuria. Hum. Mutat. 34, 927–936 (2013).
Staudigl, M., Gersting, S. W., Danecka, M. K., Messing, D. D., Woidy, M., Pinkas, D. et al. The interplay between genotype, metabolic state and cofactor treatment governs phenylalanine hydroxylase function and drug response. Hum. Mol. Genet. 20, 2628–2641 (2011).
Jennings, I. G., Cotton, R. G. & Kobe, B. Structural interpretation of mutations in phenylalanine hydroxylase protein aids in identifying genotype–phenotype correlations in phenylketonuria. Eur. J. Hum. Genet. 8, 683–696 (2000).
Desviat, L. R., Perez, B., Gamez, A., Sanchez, A., Garcia, M. J., Martinez-Pardo, M. et al. Genetic and phenotypic aspects of phenylalanine hydroxylase deficiency in Spain: molecular survey by regions. Eur. J. Hum. Genet. 7, 386–392 (1999).
Acknowledgements
We thank all patients for kindly participating in the study. This work was supported in part by NUTRICIA S.L.R. (Madrid, Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aldámiz-Echevarría, L., Llarena, M., Bueno, M. et al. Molecular epidemiology, genotype–phenotype correlation and BH4 responsiveness in Spanish patients with phenylketonuria. J Hum Genet 61, 731–744 (2016). https://doi.org/10.1038/jhg.2016.38
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2016.38
This article is cited by
-
Spectrum of PAH gene mutations in 1547 phenylketonuria patients from Iran: a comprehensive systematic review
Metabolic Brain Disease (2021)
-
Prenatal diagnosis of genetic diseases directly using paper-dried cord blood as the starting material for PCR
Analytical and Bioanalytical Chemistry (2019)