Abstract
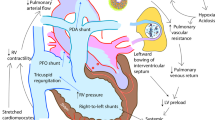
Neonatal hypoxemic respiratory failure (HRF), a deficiency of oxygenation associated with insufficient ventilation, can occur due to a variety of etiologies. HRF can result when pulmonary vascular resistance (PVR) fails to decrease at birth, leading to persistent pulmonary hypertension of newborn (PPHN), or as a result of various lung disorders including congenital abnormalities such as diaphragmatic hernia, and disorders of transition such as respiratory distress syndrome, transient tachypnea of newborn and perinatal asphyxia. PVR changes throughout fetal life, evident by the dynamic changes in pulmonary blood flow at different gestational ages. Pulmonary vascular transition at birth requires an interplay between multiple vasoactive mediators such as nitric oxide, which can be potentially inactivated by superoxide anions. Superoxide anions have a key role in the pathophysiology of HRF. Oxygen (O2) therapy, used in newborns long before our knowledge of the complex nature of HRF and PPHN, has continued to evolve. Over time has come the discovery that too much O2 can be toxic. Recommendations on the optimal inspired O2 levels to initiate resuscitation in term newborns have ranged from 100% (pre 1998) to the currently recommended use of room air (21%). Questions remain about the most effective levels, particularly in preterm and low birth weight newborns. Attaining the appropriate balance between hypoxemia and hyperoxemia, and targeting treatments to the pathophysiology of HRF in each individual newborn are critical factors in the development of improved therapies to optimize outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Clark RH . The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol 2005; 25: 251–257.
Konduri GG, Kim UO . Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am 2009; 56: 579–600.
Lakshminrusimha S . The pulmonary circulation in neonatal respiratory failure. Clin Perinatol 2012; 39: 655–683.
Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH . Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med 2001; 164: 1154–1160.
Eriksen V, Nielsen LH, Klokker M, Greisen G . Follow-up of 5- to 11-year-old children treated for persistent pulmonary hypertension of the newborn. Acta Paediatr 2009; 98: 304–309.
Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT . Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr 2002; 140: 306–310.
Rudolph A . The fetal circulation. Congenital Diseases of the Heart: Clinical-Physiological Considerations, 3rd edn. Wiley-Blackwell: Hoboken, NJ, USA, 2009, pp 1–24.
Gao Y, Raj JU . Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 2010; 90: 1291–1335.
Kiserud T . Physiology of the fetal circulation. Semin Fetal Neonatal Med 2005; 10: 493–503.
Lakshminrusimha S, Steinhorn RH . Pathophysiology of PPHN. Fetal and Neonatal Physiology, 5th edn. Elsevier: Philadelphia, PA, USA. In press (note: expected publication is July 2016. Chapter 155. ISBN 9780323352147).
Konduri GG, Gervasio CT, Theodorou AA . Role of adenosine triphosphate and adenosine in oxygen-induced pulmonary vasodilation in fetal lambs. Pediatr Res 1993; 33: 533–539.
Prsa M, Sun L, van AJ, Yoo SJ, Grosse-Wortmann L, Jaeggi E et al. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging 2014; 7: 663–670.
Lakshminrusimha S, Steinhorn RH . Pulmonary vascular biology during neonatal transition. Clin Perinatol 1999; 26: 601–619.
Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC . Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation 1996; 94: 1068–1073.
Kinsella JP, Ivy DD, Abman SH . Ontogeny of NO activity and response to inhaled NO in the developing ovine pulmonary circulation. Am J Physiol 1994; 267: H1955–H1961.
National Institutes of Health. NIH Consensus Development Conference Statement: Inhaled Nitric Oxide Therapy for Premature Infants. 27–29 October 2010.
Handley SC, Steinhorn RH, Hopper AO, Govindaswami B, Van Meurs KP, Ariagno RL et al. Patterns of Inhaled Nitric Oxide Use in California NICUs. Abstract presented at the 2015 Pediatric Academic Societies' Annual Meeting; 25–28 April 2015; San Diego, CA, USA. Abstract 2894.369.
Chow SSW, Le Marsney R, Hossain S, Haslam R, Lui K . Report of the Australian and New Zealand Neonatal Network. 2013. Report No. 18.
Ramachandrappa A, Jain L . Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol 2008; 35: 373–393.
Arcilla RA, Oh W, Lind J, Gessner IH . Pulmonary arterial pressures of newborn infants born with early and late clamping of the cord. Acta Paediatr Scand 1966; 55: 305–315.
McDonald SJ, Middleton P, Dowswell T, Morris PS . Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evid Based Child Health 2014; 9: 303–397.
Totapally B, Raju N, Perlman M . Decreased placental transfusion may lead to persistent pulmonary hypertension of the newborn (PPHN) 1156. Pediatr Res 1998; 43: 198.
Muraca MC, Negro S, Sun B, Buonocore G . Nitric oxide in neonatal hypoxemic respiratory failure. J Matern Fetal Neonatal Med 2012; 25 (Suppl 1): 47–50.
Faraci FM, Didion SP . Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 2004; 24: 1367–1373.
Saugstad OD . Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta Paediatr 1996; 85: 1–4.
Dawson TM, Dawson VL, Snyder SH . A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol 1992; 32: 297–311.
Steinhorn RH . Nitric oxide and beyond: new insights and therapies for pulmonary hypertension. J Perinatol 2008; 28 (Suppl 3): S67–S71.
Marklund SL . Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J 1984; 222: 649–655.
Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP . Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 2003; 93: 622–629.
Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res 1998; 82: 810–818.
Steinhorn RH, Morin FC III, Russell JA . The adventitia may be a barrier specific to nitric oxide in rabbit pulmonary artery. J Clin Invest 1994; 94: 1883–1888.
Lakshminrusimha S, Russell JA, Gugino SF, Ryan RM, Mathew B, Nielsen LC et al. Adjacent bronchus attenuates pulmonary arterial contractility. Am J Physiol Lung Cell Mol Physiol 2006; 291: L473–L478.
Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA et al. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 2003; 92: 683–691.
Black SM, Johengen MJ, Soifer SJ . Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 1998; 44: 821–830.
Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2005; 289: L660–L666.
Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1370–1377.
Sanderud J, Norstein J, Saugstad OD . Reactive oxygen metabolites produce pulmonary vasoconstriction in young pigs. Pediatr Res 1991; 29: 543–547.
Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH . Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 2011; 15: 1497–1506.
Keszler M, Carbone MT, Cox C, Schumacher RE . Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics 1992; 89: 670–672.
Lapointe A, Barrington KJ . Pulmonary hypertension and the asphyxiated newborn. J Pediatr 2011; 158: e19–e24.
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P . Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007; CD003311.
Groenendaal F, De Vooght KM, van BF . Blood gas values during hypothermia in asphyxiated term neonates. Pediatrics 2009; 123: 170–172.
Greisen G . Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 2005; 81: 423–428.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584.
Thoresen M . Hypothermia after perinatal asphyxia: selection for treatment and cooling protocol. J Pediatr 2011; 158: e45–e49.
Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 2014; 312: 2629–2639.
DeBoer SL, Stephens D . Persistent pulmonary hypertension of the newborn: case study and pathophysiology review. Neonatal Netw 1997; 16: 7–13.
Steinhorn RH . Neonatal pulmonary hypertension. Pediatr Crit Care Med 2010; 11: S79–S84.
Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 2000; 105: 14–20.
Little WJ . On the influence of abnormal parturition, difficult labors, premature birth, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Transactions of the Obstetrical Society of London. Longmans, Green and Co, 1861; 293.
Flagg PJ . Treatment of asphyxia in the new-born. Preliminary report of the practical application of modern scientific methods. JAMA 1928; 91: 788–791.
Obladen M . History of neonatal resuscitation. Part 2: oxygen and other drugs. Neonatology 2009; 95: 91–96.
Apgar V . A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953; 32: 260–267.
Lane N . Oxygen: the Molecule that Made the World. Oxford University Press: Oxford, England, UK, 2002.
Saugstad OD . Is oxygen more toxic than currently believed? Pediatrics 2001; 108: 1203–1205.
Howell M, Ford P . The paradoxes of a small American disaster. The beetle of Aphrodite and other medical mysteries. Random House: New York, NY, USA, 1985.
Patz A . The role of oxygen in retrolental fibroplasia. Pediatrics 1957; 19: 504–524.
Northway WH Jr, Rosan RC, Porter DY . Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276: 357–368.
Saugstad OD . Chronic lung disease: oxygen dogma revisited. Acta Paediatr 2001; 90: 113–115.
Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO . Oxygen poisoning and x-irradiation: a mechanism in common. Science 1954; 119: 623–626.
Saugstad OD . Hypoxanthine as a measurement of hypoxia. Pediatr Res 1975; 9: 158–161.
Saugstad OD, Aasen AO . Plasma hypoxanthine concentrations in pigs. A prognostic aid in hypoxia. Eur Surg Res 1980; 12: 123–129.
Aasen AO, Saugstad OD . Hypoxanthine in lethal canine endotoxin shock. Circ Shock 1979; 6: 277–283.
Frank L, Groseclose EE . Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 1984; 18: 240–244.
Saugstad OD . Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res 1988; 23: 143–150.
Saugstad OD . Oxygen radicals and pulmonary damage. Pediatr Pulmonol 1985; 1: 167–175.
Saugstad OD . Oxidative stress in the newborn—a 30-year perspective. Biol Neonate 2005; 88: 228–236.
Saugstad OD, Rootwelt T, Aalen O . Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 1998; 102: e1.
Ramji S, Ahuja S, Thirupuram S, Rootwelt T, Rooth G, Saugstad OD . Resuscitation of asphyxic newborn infants with room air or 100% oxygen. Pediatr Res 1993; 34: 809–812.
Saugstad OD, Ramji S, Soll RF, Vento M . Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008; 94: 176–182.
Saugstad OD . Resuscitation of newborn infants: from oxygen to room air. Lancet 2010; 376: 1970–1971.
World Health Organization. Guidelines on basic newborn resuscitation. http://www.who.int/maternal_child_adolescent/documents/basic_newborn_resuscitation/en/2012. Published 2012. Accessed 5 November 2015.
Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J . Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 2001; 107: 642–647.
Vento M, Sastre J, Asensi MA, Vina J . Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med 2005; 172: 1393–1398.
Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010; 122: S516–S538.
Kapadia VS, Chalak LF, DuPont TL, Rollins NK, Brion LP, Wyckoff MH . Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J Pediatr 2013; 163: 949–954.
Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009; 66: 539–544.
Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 2007; 62: 313–318.
Brown JV, Moe-Byrne T, Harden M, McGuire W . Lower versus higher oxygen concentration for delivery room stabilisation of preterm neonates: systematic review. PLoS One 2012; 7: e52033.
Saugstad OD, Aune D, Aguar M, Kapadia V, Finer N, Vento M . Systematic review and meta-analysis of optimal initial fraction of oxygen levels in the delivery room at ≤32 weeks. Acta Paediatr 2014; 103: 744–751.
Oei JL, Lui K, Wright IM et al. Targeted oxygen in the resuscitation of preterm infants and their developmental outcomes (To2rpido): a randomised controlled study. Abstract presented at the 2015 Pediatric Academic Societies Annual Meeting; April 25–28, 2015; San Diego, CA, USA. Abstract 3130.2.
Rabi Y, Lodha A, Soraisham A, Singhal N, Barrington K, Shah PS . Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation 2015; 96: 252–259.
Saugstad OD, Aune D . Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014; 105: 55–63.
Manja V, Lakshminrusimha S, Cook DJ . Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA Pediatr 2015; 169: 332–340.
Walsh MC, Di Fiore JM, Martin RJ, Gantz M, Carlo WA, Finer N . Association of oxygen target and growth status with increased mortality in small for gestational age infants: further analysis of the surfactant, positive pressure and pulse oximetry randomized trial. JAMA Pediatr 2016; 1–3.
Wollen EJ, Sejersted Y, Wright MS, Bik-Multanowski M, Madetko-Talowska A, Gunther CC et al. Transcriptome profiling of the newborn mouse lung after hypoxia and reoxygenation: hyperoxic reoxygenation affects mTOR signaling pathway, DNA repair, and JNK-pathway regulation. Pediatr Res 2013; 74: 536–544.
Acknowledgements
This article is based on discussions at a roundtable meeting supported by a grant from Mallinckrodt Pharmaceuticals, formerly Ikaria. Presentations and discussions were developed solely by the participants, without grantor input. The meeting chair Robin Steinhorn, MD, determined the agenda and attendees. SL and ODS developed the presentations and led the discussions upon which this article is based, provided critical review and revisions to the outline and manuscript drafts, provided final approval of the version to be published, and are accountable for the integrity of the content and for addressing questions. We gratefully acknowledge Robin Steinhorn, MD, for critical review of the manuscript and the contributions of the following individuals who participated in discussion that shaped the content of this article: Namasivayam Ambalavanan, MD; Judy L Aschner, MD; Jason Gien, MD; John Kinsella, MD; G Ganesh Konduri, MD; Robin Steinhorn, MD. Writing and editorial assistance was provided by Saema Magre, Sharon Suntag and Julie Gerke of Quintiles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SL and ODS received honoraria for their participation in a roundtable meeting supported by a grant from Mallinckrodt Pharmaceuticals, formerly Ikaria. SL was a member of the speaker’s bureau for Ikaria from June 2010 to October 2014 and has received grant support from the American Academy of Pediatrics and Canadian Pediatric Society. SL also received an NIH grant (5R01HD072929-03).
Rights and permissions
About this article
Cite this article
Lakshminrusimha, S., Saugstad, O. The fetal circulation, pathophysiology of hypoxemic respiratory failure and pulmonary hypertension in neonates, and the role of oxygen therapy. J Perinatol 36 (Suppl 2), S3–S11 (2016). https://doi.org/10.1038/jp.2016.43
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jp.2016.43
This article is cited by
-
Care of the critically ill neonate with hypoxemic respiratory failure and acute pulmonary hypertension: framework for practice based on consensus opinion of neonatal hemodynamics working group
Journal of Perinatology (2022)
-
Renal biomarkers of acute kidney injury in response to increasing intermittent hypoxia episodes in the neonatal rat
BMC Nephrology (2021)
-
Effect of continuous positive airway pressure versus nasal cannula on late preterm and term infants with transient tachypnea of the newborn
Journal of Perinatology (2021)
-
Perioperative management of arteriovenous malformation guided by integrated evaluation of hemodynamics
European Journal of Pediatrics (2021)
-
Venovenous versus venoarterial extracorporeal membrane oxygenation among infants with hypoxic-ischemic encephalopathy: is there a difference in outcome?
Journal of Perinatology (2021)