Abstract
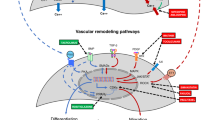
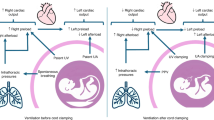
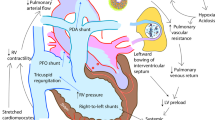
Future priorities for the management of hypoxemic respiratory failure (HRF) and pulmonary hypertension include primary prevention of neonatal lung diseases, ‘precision medicine’ and translating promising clinical and preclinical research into novel therapies. Promising areas of investigation include noninvasive ventilation strategies, emerging pulmonary vasodilators (for example, cinaciguat, intravenous bosentan, rho-kinase inhibitors, peroxisome proliferator-activated receptor-γ agonists) and hemodynamic support (arginine vasopressin). Research challenges include the optimal timing for primary prevention interventions and development of validated biomarkers that predict later disease or serve as surrogates for long-term respiratory outcomes. Differentiating respiratory disease endotypes using biomarkers and experimental therapies tailored to the underlying pathobiology are central to the concept of ‘precision medicine’ (that is, prevention and treatment strategies that take individual variability into account). The ideal biomarker should be expressed early in the neonatal course to offer an opportunity for effective and targeted interventions to modify outcomes. The feasibility of this approach will depend on the identification and validation of accurate, rapid and affordable point-of-care biomarker tests. Trials targeting patient-specific pathobiology may involve less risk than traditional randomized controlled trials that enroll all at-risk neonates. Such approaches would reduce trial costs, potentially with fewer negative trials and improved health outcomes. Initiatives such as the Prematurity and Respiratory Outcomes Program, supported by the National Heart, Lung, and Blood Institute, provide a framework to develop refined outcome measures and early biomarkers that will enhance our understanding of novel, mechanistic therapeutic targets that can be tested in clinical trials in neonates with HRF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Roberts JD Jr, Fineman JR, Morin FC III, Shaul PW, Rimar S, Schreiber MD et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 1997; 336: 605–610.
Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Pediatrics 1997; 99: 838–845.
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 2000; 342: 469–474.
Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. The Franco-Belgium Collaborative NO Trial Group. Lancet 1999; 354: 1066–1071.
Gonzalez A, Fabres J, D'Apremont I, Urcelay G, Avaca M, Gandolfi C et al. Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. J Perinatol 2010; 30: 420–424.
Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics 2004; 113: 559–564.
Konduri GG, Sokol GM, Van Meurs KP, Singer J, Ambalavanan N, Lee T et al. Impact of early surfactant and inhaled nitric oxide therapies on outcomes in term/late preterm neonates with moderate hypoxic respiratory failure. J Perinatol 2013; 33: 944–949.
Findlay RD, Taeusch HW, Walther FJ . Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996; 97: 48–52.
Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH . Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr 1998; 132: 40–47.
McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL . Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 2014; 11 Suppl 3: S146–S153.
Rubenfeld GD . Confronting the frustrations of negative clinical trials in acute respiratory distress syndrome. Ann Am Thorac Soc 2015; 12 Suppl 1: S58–S63.
Armitage P . Sequential Medical Trials, 2nd ed. New York, USA: John Wiley & Sons, 1975.
Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB . Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics 1985; 76: 479–487.
Carlo WA, Siner B, Chatburn RL, Robertson S, Martin RJ . Early randomized intervention with high-frequency jet ventilation in respiratory distress syndrome. J Pediatr 1990; 117: 765–770.
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM . Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988; 82: 527–532.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114: 1305–1311.
Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A et al. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol 2015; 35: 313–321.
Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc 2015; 12: 1822–1830.
Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr 2015; 15: 37.
University of Pennsylvania; National Heart, Lung, and Blood Institute. Prematurity and Respiratory Outcomes Program (PROP). In: Clinicaltrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US), 2011 [cited 2 Dec 2015]. Available from https://www.clinicaltrials.gov/ct2/show/NCT01435187?term=NCT01435187&rank=1 NLM. Identifier: NCT01435187.
Collins FS, Varmus H . A new initiative on precision medicine. N Engl J Med 2015; 372: 793–795.
Palma JP, Benitz WE, Tarczy-Hornoch P, Butte AJ, Longhurst CA . Neonatal informatics: transforming neonatal care through translational bioinformatics. Neoreviews 2012; 13: e281–e284.
Fike CD, Summar M, Aschner JL . L-citrulline provides a novel strategy for treating chronic pulmonary hypertension in newborn infants. Acta Paediatr 2014; 103: 1019–1026.
Fike CD, Dikalova A, Kaplowitz MR, Cunningham G, Summar M, Aschner JL . Rescue treatment with L-citrulline inhibits hypoxia-induced pulmonary hypertension in newborn pigs. Am J Respir Cell Mol Biol 2015; 53: 255–264.
Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 2011; 17: 1619–1626.
Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A et al. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol 2008; 65: 51–59.
Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G et al. L-citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 2009; 297: L506–L511.
Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M et al. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res 2010; 68: 519–525.
Keller RL, Tacy TA, Hendricks-Munoz K, Xu J, Moon-Grady AJ, Neuhaus J et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med 2010; 182: 555–561.
Büssemaker E, Pistrosch F, Förster S, Herbrig K, Gross P, Passauer J et al. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol 2007; 293: H541–H547.
Chapados R, Abe K, Ihida-Stansbury K, McKean D, Gates AT, Kern M et al. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res 2006; 99: 837–844.
Parker TA, Roe G, Grover TR, Abman SH . Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2006; 291: L976–L982.
Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA . Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2001; 25: 628–635.
Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 2007; 100: 923–929.
Löhn M, Steioff K, Bleich M, Busch AE, Ivashchenko Y . Inhibition of Rho-kinase stimulates nitric oxide-independent vasorelaxation. Eur J Pharmacol 2005; 507: 179–186.
Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y et al. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 2006; 48: 280–285.
Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V et al. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 2007; 50: 697–702.
Gien J, Tseng N, Seedorf G, Roe G, Abman SH . Endothelin-1 impairs angiogenesis in vitro through Rho-kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatr Res 2013; 73: 252–262.
Gien J, Tseng N, Seedorf G, Roe G, Abman SH . Peroxisome proliferator activated receptor-gamma-Rho-kinase interactions contribute to vascular remodeling after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014; 306: L299–L308.
Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 2009; 296: L1031–L1041.
Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B et al. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 2011; 301: L125–L134.
Steinhorn RH, Russell JA, Morin FC III . Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol 1995; 268: H1483–H1489.
Chester M, Seedorf G, Tourneux P, Gien J, Tseng N, Grover T et al. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2011; 301: L755–L764.
Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 2006; 174: 1370–1377.
Tessler RB, Zadinello M, Fiori H, Colvero M, Belik J, Fiori RM . Tadalafil improves oxygenation in a model of newborn pulmonary hypertension. Pediatr Crit Care Med 2008; 9: 330–332.
Acker SN, Kinsella JP, Abman SH, Gien J . Vasopressin improves hemodynamic status in infants with congenital diaphragmatic hernia. J Pediatr 2014; 165: 53–58.
Acknowledgements
This article is based on discussions at a roundtable meeting supported by a grant from Mallinckrodt Pharmaceuticals, formerly Ikaria. Presentations and discussions were developed solely by the participants, without grantor input. The meeting chair (RHS) determined the agenda and attendees. JLA, JG, NA, JPK, GGK, SL, ODS and RHS developed the presentations and led the discussions upon which this article is based. Participants provided critical review and revisions to the outline and manuscript drafts, provided final approval of the version to be published, and are accountable for the integrity of the content and for addressing questions. Writing and editorial assistance was provided by John Kross, and Sharon Suntag and Julie Gerke of Quintiles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JLA, JG, NA, JPK, GGK, SL, ODS and RHS received honoraria for their participation in a roundtable meeting supported by a grant from Mallinckrodt Pharmaceuticals, formerly Ikaria. JLA is named on an intellectual property rights patent for the use of intravenous citrulline for neonatal lung diseases. NA has received research support from Pfizer and has received research support as a mentor from Ikaria. GGK has received consulting fees from Boston Health Economics and Actelion Clinical Research. SL was a member of the speaker’s bureau for Ikaria from June 2010 to October 2014 and has received grant support from the American Academy of Pediatrics and Canadian Pediatric Society. RHS has received research grant support from Pfizer. NIH Grants: 1U01HL101456 (JLA); U01 HL122626 and R01 HD067126 (NA); 5R01HD072929-03 (SL); 5R01HL057268-11 (GGK); 5R01HL054705-13 (RHS).
Rights and permissions
About this article
Cite this article
Aschner, J., Gien, J., Ambalavanan, N. et al. Challenges, priorities and novel therapies for hypoxemic respiratory failure and pulmonary hypertension in the neonate. J Perinatol 36 (Suppl 2), S32–S36 (2016). https://doi.org/10.1038/jp.2016.47
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jp.2016.47
This article is cited by
-
Cost-effectiveness analysis of pulse oximetry screening for critical congenital heart defects following homebirth and early discharge
European Journal of Pediatrics (2019)