Abstract
Objective:
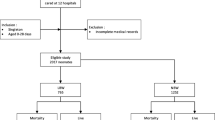
The excipients benzyl alcohol, propylene glycol and ethanol are present in medications used in the neonatal intensive care unit. Exposure to high levels can have adverse effects in a neonatal population. The objective was to quantify excipient exposure in very low birth weight (VLBW) neonates and identify risk factors associated with greater exposure.
Study design:
A retrospective record review of VLBW infants admitted over 1 year. Excipient exposures were calculated and multivariable regression analyses identified risk factors for increasing exposure.
Results:
In total, 98% of subjects were exposed to at least one excipient. A total of 5 to 9% received doses higher than recommended for adults. Necrotizing enterocolitis, seizure, bronchopulmonary dysplasia and longer stay predicted higher excipient exposure.
Conclusion:
The excipients examined are in medications commonly prescribed for VLBW neonates, and cumulative doses may exceed recommended exposures for adults. Although safety profiles have not been established, judicious use of medication containing these excipients is warranted for this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Shehab N, Lewis CL, Streetman DD, Donn SM . Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med 2009; 10 (2): 256–259.
Whittaker A, Currie AE, Turner MA, Field DJ, Mulla H, Pandya HC . Toxic additives in medication for preterm infants. Arch Dis Child Fetal Neonatal Ed 2009; 94 (4): F236–F240.
Souza A Jr, Santos D, Fonseca S, Medeiros M, Batista L, Turner M et al. Toxic excipients in medications for neonates in brazil. Eur J Pediatr 2014; 173 (7): 935–945.
Nellis G, Metsvaht T, Varendi H, Toompere K, Lass J, Mesek I et al. Potentially harmful excipients in neonatal medicines: a pan-european observational study. Arch Dis Child 2015; 100 (7): 694–699.
LeBel M, Ferron L, Masson M, Pichette J, Carrier C . Benzyl alcohol metabolism and elimination in neonates. Dev Pharmacol Ther 1988; 11 (6): 347–356.
McCloskey SE, Gershanik JJ, Lertora JJ, White L, George WJ . Toxicity of benzyl alcohol in adult and neonatal mice. J Pharm Sci 1986; 75 (7): 702–705.
Gershanik J, Boecler B, Ensley H, McCloskey S, George W . The gasping syndrome and benzyl alcohol poisoning. N Engl J Med 1982; 307 (22): 1384–1388.
Hiller JL, Benda GI, Rahatzad M, Allen JR, Culver DH, Carlson CV et al. Benzyl alcohol toxicity: Impact on mortality and intraventricular hemorrhage among very low birth weight infants. Pediatrics 1986; 77 (4): 500–506.
Menon PA, Thach BT, Smith CH, Landt M, Roberts JL, Hillman RE et al. Benzyl alcohol toxicity in a neonatal intensive care unit. incidence, symptomatology, and mortality. Am J Perinatol 1984; 1 (4): 288–292.
Brown WJ, Buist NR, Gipson HT, Huston RK, Kennaway NG . Fatal benzyl alcohol poisoning in a neonatal intensive care unit. Lancet 1982; 1 (8283): 1250.
MacDonald MG, Getson PR, Glasgow AM, Miller MK, Boeckx RL, Johnson EL . Propylene glycol: Increased incidence of seizures in low birth weight infants. Pediatrics 1987; 79 (4): 622–625.
American Academy of Pediatrics Committee on Drugs. "Inactive" ingredients in pharmaceutical products: Update (subject review) Pediatrics 1997; 99 (2): 268–278.
Wilson KC, Reardon C, Theodore AC, Farber HW . Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: A case series and prospective, observational pilot study. Chest 2005; 128 (3): 1674–1681.
Glasgow AM, Boeckx RL, Miller MK, MacDonald MG, August GP, Goodman SI . Hyperosmolality in small infants due to propylene glycol. Pediatrics 1983; 72 (3): 353–355.
Murch S, Costeloe K . Hyperosmolality related to propylene glycol in an infant. BMJ 1990; 301 (6748): 389.
Tran MN, Wu AH, Hill DW . Alcohol dehydrogenase and catalase content in perinatal infant and adult livers: Potential influence on neonatal alcohol metabolism. Toxicol Lett 2007; 169 (3): 245–252.
Food and Agriculture Organization of the United Nations/World Health Organization Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA). In: International Life Sciences Institute: Washington DC, USA, 1994.
Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives, Wld Hlth Org. techn. Rep. Ser., 1974, No. 539; FAO Nutrition Meetings Report Series, 1974, No. 53. Available at Http://Www.inchem.org/documents/jecfa/jecmono/v05je90.htm[Internet].; 1974.
Nair B . Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol 2001; 20 (Suppl 3): 23–50.
Williams JF, Smith VC, COMMITTEE ON SUBSTANCE ABUSE. Fetal alcohol spectrum disorders. Pediatrics 2015; 136 (5): e1395–e1406.
American Academy of Pediatrics Committee on Drugs. Ethanol in liquid preparations intended for children. Pediatrics 1984; 73 (3): 405–407.
Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ . Births: final data for 2014. Natl Vital Stat Rep 2015; 64 (12): 1–64.
Kliegman RM, Walsh MC . Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987; 17 (4): 213–288.
Richardson DK, Corcoran JD, Escobar GJ, Lee SK . SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J Pediatr 2001; 138 (1): 92–100.
Dorling JS, Field DJ, Manktelow B . Neonatal disease severity scoring systems. Arch Dis Child Fetal Neonatal Ed 2005; 90 (1): F11–F16.
Lass J, Naelapaa K, Shah U, Kaar R, Varendi H, Turner MA et al. Hospitalised neonates in estonia commonly receive potentially harmful excipients. BMC Pediatr 2012; 12 (136): 2431–12-136.
Allegaert K, Vanhaesebrouck S, Kulo A, Cosaert K, Verbesselt R, Debeer A et al. Prospective assessment of short-term propylene glycol tolerance in neonates. Arch Dis Child 2010; 95 (12): 1054–1058.
Pandya HC, Mulla H, Hubbard M, Cordell RL, Monks PS, Yakkundi S et al. Essential medicines containing ethanol elevate blood acetaldehyde concentrations in neonates. Eur J Pediatr 2016; 175 (6): 841–847.
Arant BS Jr. . Postnatal development of renal function during the first year of life. Pediatr Nephrol 1987; 1 (3): 308–313.
De Cock RF, Knibbe CA, Kulo A, de Hoon J, Verbesselt R, Danhof M et al. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol 2013; 75 (1): 162–171.
Acknowledgements
We gratefully acknowledge the assistance of Dr Nakia Eldridge Pharm. D. and Dr Omayma Kish Pharm. D. who helped with determining excipient concentrations, and Dr. Elizabeth Powell PhD and Dr Rose Viscardi M.D. who gave advice throughout this study. SMM receives funding from NIH: NIAAA AA022413, AA018693, AA006916 and AA017823. CFB receives funding from NIH: NICHD HD085928, HD085061 and the Gerber Foundation. Funding organizations had no role the design of the study, collection and analysis of data or decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Akinmboni, T., Davis, N., Falck, A. et al. Excipient exposure in very low birth weight preterm neonates. J Perinatol 38, 169–174 (2018). https://doi.org/10.1038/jp.2017.165
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/jp.2017.165
This article is cited by
-
Weighing up the evidence on ethanol excipient use in common neonatal medications
Pediatric Research (2025)
-
Ethanol metabolites increase over time in preterm infants receiving ethanol containing medications: a concern
Pediatric Research (2025)
-
Environmental exposures in the neonatal intensive care unit impacting neurodevelopmental outcomes for neonates
Pediatric Research (2025)
-
Recessive Prevalence of Potentially Harmful Excipients Used for Hospitalized Neonates in a Neonatal Intensive Care Unit: a Retrospective Study
Pharmaceutical Chemistry Journal (2025)
-
Quantitative Investigation on Exposure to Potentially Harmful Excipients by Injection Drug Administration in Children Under 2 Years of Age and Analysis of Association with Adverse Events: A Single-Center, Retrospective Observational Study
Therapeutic Innovation & Regulatory Science (2024)