Abstract
Objective:
To identify characteristics associated with undergoing cell-free DNA (cfDNA) and multiple marker screening (MMS) simultaneously or redundantly (after receiving negative results from the first screening test) among women aged ⩾35 years.
Study design:
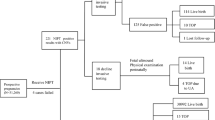
Participants presenting for prenatal testing completed a questionnaire that included measures of pregnancy worry and attitudes toward potential testing outcomes; data on prenatal test use was obtained via medical record review. We used multivariable logistic regression to identify factors associated with redundant or simultaneous screening.
Results:
Among 164 participants, 69 (42.1%) had cfDNA redundantly (n=51) to, or simultaneously (n=18) with, MMS. Compared with the 46 MMS-negative women who did not undergo further testing, those who underwent redundant or simultaneous cfDNA/MMS screening were more likely to have annual family incomes >$150 000, to feel having a miscarriage would be worse than having an intellectually disabled child, to desire comprehensive testing for intellectual disability and to have more pregnancy worry.
Conclusion:
Providers who counsel patients on prenatal aneuploidy screening tests should explain the appropriate utilization of these screening tests to avoid unnecessary or minimally informative use of multiple tests.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Practice Bulletin No. 640. Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol 2015; 126: e31–e37.
Norton ME, Brar H, Weiss J, Karimi A, Laurent LC, Caughey AB et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012; 207 (137): e1–e8.
Porreco RP, Garite TJ, Maurel K, Maurel K, Marusiak B, et alObstetrix Collaborative Research Network. Noninvasive prenatal screening for fetal trisomies 21, 18, 13, and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol 2014; 211 (365): e1–e12.
Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015; 372: 1589–1597.
Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH . Analysis of cell-free DNA in maternal blood screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2015; 45: 249–266.
Benn P, Cuckle H, Pergament E . Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound Obstet Gynecol 2013; 42: 15–33.
Verweij EJ, de Boer MA, Oepkes D . Non-invasive prenatal testing for trisomy 13: more harm than good? Ultrasound Obstet Gynecol 2014; 44: 112–114.
Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 2014; 370: 799–808.
Ariosa Diagnostics & The Harmony Prenatal Test. “For expecting parents.” [Internet]. United States; 2015 – [Cited 5 September 2016]. Available at http://www.ariosadx.com/expecting-parents.
Sequenom: MaterniT:21PLUS. “For expecting parents: Overview.” [Internet]. United States; 2015 – [Cited 5 September 2016]. Available at https://www.sequenom.com/tests/reproductive-health/maternit21-plus#patient-overview.
Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med 2016; 18: 1056–1065.
Society for Maternal-Fetal Medicine (SMFM) Publication Committee. #36: Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol 2015; 212: 711–716.
Society for Maternal-Fetal Medicine (SMFM) Publication Committee. SMFM Statement: clarification of recommendations regarding cell-free DNA aneuploidy screening. Am J Obstet Gynecol 2015; 213: 753–754.
Devers PL, Cronister A, Ormond KE, Facio F, Brasington CK, Flodman P . Noninvasive prenatal testing/noninvasive prenatal diagnosis: the Position of the National Society of Genetic Counselors. J Genet Couns 2013; 22: 291–295.
Benn P, Borell A, Chiu R, Cuckle H, Dugoff L, Faas B et al. Position Statement from the Aneuploidy Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn 2013; 33: 622–629.
Taylor JB, Chock VY, Hudgins L . NIPT in a clinical setting: an analysis of uptake in the first months of clinical availability. J Genet Couns 2014; 23: 72–78.
Larion S, Warsof SL, Romary L, Mlynarczyk M, Peleg D, Abuhamad AZ . Uptake of noninvasive prenatal testing at a large academic referral center. Am J Obstet Gynecol 2014; 211 (651): e1–e7.
Kuppermann M, Learman LA, Gates E, Gregorich SE, Nease RF Jr, Lewis J et al. Beyond race or ethnicity and socioeconomic status: predictors of prenatal testing for Down syndrome. Obstet Gynecol 2006; 107 (1087-97): 21.
Kuppermann M, Pena S, Bishop J, Nakagawa S, Gregorich SE, Sit A et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized controlled trial. JAMA 2014; 312: 1210–1217.
Farrell R, Hawkins A, Barragan D, Hudgins L, Taylor J . Knowledge, understanding, and uptake of noninvasive prenatal testing among Latina women. Prenat Diagn 2015; 35: 748–753.
Kuppermann M, Gates E, Washington AE . Racial-ethnic differences in prenatal diagnostic test use and outcomes: preferences, socioeconomics, or patient knowledge? Obstet Gynecol 1996; 87: 675–682.
HealthNet National Medical Policy #NMP506: MaterniT21 PLUS, Harmony, Verifi, or Panorama Prenatal Testing, Effective Date: December 2011, updated October 2015 [Internet]. United States; 2015 – [Cited 5 February 2016]. Available at https://www.healthnet.com/portal/provider/content/iwc/provider/unprotected/working_with_HN/content/medical_policies.action.
The California Prenatal Screening Program and Genetic Disease Screening Program: Prenatal Screening Program Changes, Effective November 2013: Prenatal Screening Program Adds Non-invasive Prenatal Testing (NIPT) as an optional PDC follow-up service [Internet]. United States; 2013 – [Cited 5 February 2016]. Available at http://www.cdph.ca.gov/programs/GDSP/Documents/Newletter%20nov%20%202013.pdf.
Choosing Wisely (An Initiative of the ABIM Foundation) and Society for Maternal-Fetal Medicine: Five Things Physicians and Patients Should Question and Five More Things Physicians and Patients Should Question [Internet]. United States; February 1, 2016 [Cited 8 February 2016]. Available at http://www.choosingwisely.org/wp-content/uploads/2015/02/SMFM-Choosing-Wisely-List.pdf.
Acknowledgements
We thank Michelle Moghadassi, MPH, and Sanae Nakagawa, MA, for their assistance with data analysis. This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
From 1 April 2012 through 31 March 2014, MK was the UCSF site PI for a clinical study of cfDNA testing among average-risk women (the Noninvasive Examination of Trisomy NEXT Study) funded by Ariosa Diagnostics, and her institution received unrestricted research funding for her research program from Natera in 2013.
Additional information
Components of this work were presented as a poster on 11 February 2016 at the Society of Maternal Fetal Medicine 36th Annual Meeting in Atlanta, GA, USA (Abstract 325).
Appendix 1
Appendix 1

Rights and permissions
About this article
Cite this article
Lewkowitz, A., Kaimal, A., Thao, K. et al. Sociodemographic and attitudinal predictors of simultaneous and redundant multiple marker and cell-free DNA screening among women aged ⩾35 years. J Perinatol 37, 772–777 (2017). https://doi.org/10.1038/jp.2017.66
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jp.2017.66