Abstract
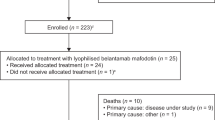
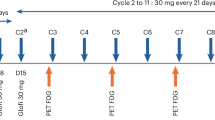
We conducted a phase II, noncomparative, open-label, multicenter GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) study (CLL0809) to assess the efficacy and safety of bendamustine in combination with ofatumumab (BendOfa) in relapsed/refractory chronic lymphocytic leukemia (CLL). Forty-seven patients from 14 centers were evaluated. Therapy consisted of bendamustine (70 mg/m2) for 2 consecutive days every 28 days, and ofatumumab 300 mg on day 1 and 1000 mg on day 8 during the first cycle, and 1000 mg on day 1 subsequently. Treatment was administered up to six cycles. The overall response rate (ORR), as per intention-to-treat analysis, was 72.3% (95% confidence of interval (CI), 57–84%), with 17% complete responses. After a median follow-up of 24.2 months, the overall survival was 83.6% (95% CI, 73.0–95.7%) and the progression-free survival (PFS) was 49.6% (95% CI, 35.9–68.6%). The median PFS was 23.6 months. Univariate and multivariate analyses were used to identify clinical and biological characteristics associated with ORR and PFS. Myelosuppression was the most common toxicity; grade ⩾3 neutropenia was observed in 61.7% of patients; however, grade ⩾3 infections occurred in 6% of patients. BendOfa is feasible and effective in relapsed/refractory CLL patients, including patients with high-risk clinical and biological features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dhner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456.
Dhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1910–1916.
Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999; 94: 1840–1847.
Pospisilova S, Gonzalez D, Malcikova J, Trbusek M, Rossi D, Kater AP et al. ERIC recommendations on TP53 mutation analysis in chronic lymphocytic leukemia. Leukemia 2012; 26: 1458–1461.
Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003; 348: 1764–1775.
Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011; 475: 101–105.
Rossi D, Bruscaggin A, Spina V, Rasi S, Khiabanian H, Messina M et al. Mutations of the SF3B1 splicing factor in chronic lymphocytic leukemia: association with progression and fludarabine-refractoriness. Blood 2011; 118: 6904–6908.
Rossi D, Rasi S, Fabbri G, Spina V, Fangazio M, Forconi F et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood 2012; 119: 521–529.
Rossi D, Fangazio M, Rasi S, Vaisitti T, Monti S, Cresta S et al. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood 2012; 119: 2854–2862.
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1750–1757.
Flinn I, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol 2007; 25: 793–798.
Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006; 107: 885–891.
Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet 2007; 370: 230–239.
Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4070–4078.
Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 2011; 117: 3016–3024.
Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1756–1765.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008; 112: 975–980.
Keating MJ, O’Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma 2002; 43: 1755–1762.
Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009; 27: 4378–4384.
Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2011; 29: 3559–3566.
Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1749–1755.
Wierda WG, Padmanabhan S, Chan GW, Stilgenbauer S, Williams CD, Hellmann A et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011; 118: 5126–5129.
Rawstron AC, Villamor N, Ritgen M, Böttcher S, Ghia P, Zehnder JL et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007; 21: 956–964.
Bottcher S, Stilgenbauer S, Busch R, Brüggemann M, Raff T, Pott C et al. Standardized MRD flow and ASO IGH RQ-PCR for MRD quantification in CLL patients after rituximab containing immunochemotherapy: A comparative analysis. Leukemia 2009; 23: 2007–2017.
National Cancer InstituteCommon Terminology Criteria for Adverse Events v. 4.0 (CTCAE) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Simon R . Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10.
Ferrajoli A, Falchi L, O'Brien S, Wierda W, Faderl S, Smith SC et al. Combination of Ofatumumab and Lenalidomide in Patients with Relapsed Chronic Lymphocytic Leukemia (CLL). Results of a Phase II Trial. Blood (ASH Annual Meeting Abstracts) 2012; 120: 720.
Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 2012; 30: 980–988.
Acknowledgements
We thank the clinicians, nurses and data managers of the 14 institutions who entered their patients into the GIMEMA CLL0809 study and provided the necessary data to make this study possible. In addition, we are grateful to those who spent their time to quality-control tasks. Finally, the study could not have been a success without the continuous efforts of the members of the GIMEMA data center.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
FZ received compensation as a member of advisory board of Roche, GlaxoSmithKline, Mundipharma and AMGEN Biotech. FM received honoraria from Mundipharma and GlaxoSmithKline. MG received compensation as a member of the advisory board by Mundipharma and GlaxoSmithKline. DR received honoraria from GlaxoSmithKline. RF received compensation by GlaxoSmithKline and Mundipharma as expert testimony. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cortelezzi, A., Sciumè, M., Liberati, A. et al. Bendamustine in combination with Ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia 28, 642–648 (2014). https://doi.org/10.1038/leu.2013.334
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/leu.2013.334
Keywords
This article is cited by
-
Current Treatment of Chronic Lymphocytic Leukemia
Current Treatment Options in Oncology (2017)
-
Chemotherapy plus Ofatumumab at Standard or Mega dose in relapsed CLL (COSMIC) trial: study protocol for a phase II randomised controlled trial
Trials (2016)
-
Human organic cation transporter 1 (hOCT1) as a mediator of bendamustine uptake and cytotoxicity in chronic lymphocytic leukemia (CLL) cells
The Pharmacogenomics Journal (2015)
-
The efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome: A meta-analysis
Scientific Reports (2015)
-
Targeted Therapy for Chronic Lymphocytic Leukemia: Current Status and Future Directions
Drugs (2015)