Abstract
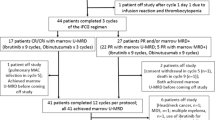
ADMIRE was a multicenter, randomized-controlled, open, phase IIB superiority trial in previously untreated chronic lymphocytic leukemia. Conventional front-line therapy in fit patients is fludarabine, cyclophosphamide and rituximab (FCR). Initial evidence from non-randomized phase II trials suggested that the addition of mitoxantrone to FCR (FCM-R) improved remission rates. Two hundred and fifteen patients were recruited to assess the primary end point of complete remission (CR) rates according to International Workshop on Chronic Lymphocytic Leukemia criteria. Secondary end points were progression-free survival (PFS), overall survival (OS), overall response rate, minimal residual disease (MRD) negativity and safety. At final analysis, CR rates were 69.8 FCR vs 69.3% FCM-R (adjusted odds ratio (OR): 0.97; 95% confidence interval (CI): (0.53–1.79), P=0.932). MRD-negativity rates were 59.3 FCR vs 50.5% FCM-R (adjusted OR: 0.70; 95% CI: (0.39–1.26), P=0.231). During treatment, 60.0% (n=129) of participants received granulocyte colony-stimulating factor as secondary prophylaxis for neutropenia, a lower proportion on FCR compared with FCM-R (56.1 vs 63.9%). The toxicity of both regimens was acceptable. There are no significant differences between the treatment groups for PFS and OS. The trial demonstrated that the addition of mitoxantrone to FCR did not increase the depth of response. Oral FCR was well tolerated and resulted in impressive responses in terms of CR rates and MRD negativity compared with historical series with intravenous chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016; 127: 208–215.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008; 112: 975–980.
Wilder DD, Ogden JL, Jain VK . Efficacy of fludarabine/mitoxantrone/dexamethasone alternating with CHOP in bulky follicular non-Hodgkin's lymphoma. Clin Lymphoma 2002; 2: 229–237.
Zinzani PL, Magagnoli M, Moretti L, Battista R, Ronconi F, De Renzo A et al. Fludarabine based chemotherapy in untreated mantle cell lymphomas: an encouraging experience in 29 patients. Haematologica 1999; 84: 1002–1006.
Bosch F, Ferrer A, Villamor N, Gonzalez M, Briones J, Gonzalez-Barca E et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: High response rate and disease eradication. Clin Cancer Res 2008; 14: 155–161.
Bosch F, Abrisqueta P, Villamor N, Terol MJ, Gonzalez-Barca E, Ferra C et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol 2009; 27: 4578–4584.
Bosch F, Ferrer A, Lopez-Guillermo A, Gine E, Bellosillo B, Villamor N et al. Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukaemia. Br J Haematol 2002; 119: 976–984.
Hendry L, Bowen A, Matutes E, Swansbury J, Catovsky D . Fludarabine, cyclophosphamide and mitoxantrone in relapsed or refractory chronic lymphocytic leukemia and low grade non-Hodgkin's lymphoma. Leuk Lymphoma 2004; 45: 945–950.
Hillmen P, Cohen DR, Cocks K, Pettitt A, Sayala HA, Rawstron AC et al. A randomized phase II trial of fludarabine, cyclophosphamide and mitoxantrone (FCM) with or without rituximab in previously treated chronic lymphocytic leukaemia. Br J Haematol 2011; 152: 570–578.
Kwok M, Rawstron AC, Varghese A, Hillmen P . Minimal residual disease is a predictor for progression-free and overall survival in chronic lymphocytic leukemia (CLL) that is independent of the type or line of therapy. Blood 2009; 114: 226.
Rawstron AC, Kennedy B, Evans PAS, Davies FE, Richards SJ, Haynes AP et al. Quantitation of minimal disease levels in chronic lymphocytic leukemia using a sensitive flow cytometric assay improves the prediction of outcome and can be used to optimize therapy. Blood 2001; 98: 29–35.
Rawstron AC, Villamor N, Ritgen M, Boettcher S, Ghia P, Zehnder JL et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007; 21: 956–964.
Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 2012; 30: 980–988.
Kwok M, Rawstron A, Varghese A, Evans P, O'Connor S, Doughty C et al. Independent prognostic significance of minimal residual disease status in chronic lymphocytic leukaemia. Lancet 383: S66.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456.
Dearden CE, Richards S, Else M, Catovsky D, Hillmen P . A comparison of the efficacy and safety of oral and intravenous fludarabine in chronic lymphocytic leukemia in the LRF CLL4 trial. Cancer 2011; 117: 2452–2460.
Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events 2006. v3.0. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Royston P, Parmar MKB . The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med 2011; 30: 2409–2421.
Moher D, Schulz KF, Altman DG, Grp C . The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001; 357: 1191–1194.
Thompson PA, Tam CS, O'Brien SM, Wierda WG, Stingo F, Plunkett W et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 2016; 127: 303–309.
Howard DR, Munir T, McParland L, Rawstron AC, Milligan D, Schuh A et al. Results of the randomized phase IIB ARCTIC trial of low dose rituximab in previously untreated CLL. Leukaemia 2017; e-pub ahead of print 24 March 2017; doi:10.1038/leu.2017.96.
Acknowledgements
ADMIRE is an National Institute for Health Research (NIHR) Portfolio Study developed in association with the NCRI CLL Subgroup and funded by Roche Products Limited. The views and opinions expressed there in are those of the authors and do not necessarily reflect those of Roche, NIHR or the NHS. The authors thank all patients and hospital staff who contributed to this study. In addition, they acknowledge the invaluable support provided by the independent DMC and TSC and Roche Products Limited for providing rituximab for the trial as well as an unrestricted grant to support the running of the trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor Hillmen received research funding and speakers’ fees from Roche Products Limited. Dr Rawstron, Dr Munir, Dr Fegan and Dr Hamblin reports personal fees from Roche Products Limited. Dr Bloor has also reported personal, consultancy/advisory and speaker’s fees from Roche Products Limited. Professor Gribben reports personal fees and expenses from Roche Products Limited. The other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Munir, T., Howard, D., McParland, L. et al. Results of the randomized phase IIB ADMIRE trial of FCR with or without mitoxantrone in previously untreated CLL. Leukemia 31, 2085–2093 (2017). https://doi.org/10.1038/leu.2017.65
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/leu.2017.65
This article is cited by
-
Combined analysis of IGHV mutations, telomere length and CD49d identifies long-term progression-free survivors in TP53 wild-type CLL treated with FCR-based therapies
Leukemia (2022)
-
Whole-genome sequencing of chronic lymphocytic leukemia identifies subgroups with distinct biological and clinical features
Nature Genetics (2022)
-
Measurable residual disease in chronic lymphocytic leukemia: expert review and consensus recommendations
Leukemia (2021)
-
Telomere length predicts for outcome to FCR chemotherapy in CLL
Leukemia (2019)
-
Results of the randomized phase IIB ARCTIC trial of low-dose rituximab in previously untreated CLL
Leukemia (2017)