Abstract
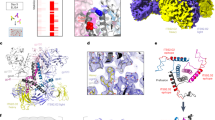
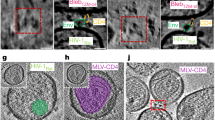
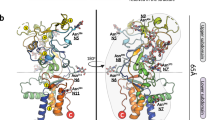
Envelope glycoprotein (Env) spikes on AIDS retroviruses initiate infection of host cells and are therefore targets for vaccine development. Though crystal structures for partial Env subunits are known, the structure and distribution of native Env spikes on virions is obscure. We applied cryoelectron microscopy tomography to define ultrastructural details of spikes. Virions of wild-type human immunodeficiency virus 1 (HIV-1) and a mutant simian immunodeficiency virus (SIV) had ∼14 and ∼73 spikes per particle, respectively, with some clustering of HIV-1 spikes. Three-dimensional averaging showed that the surface glycoprotein (gp120) ‘head’ of each subunit of the trimeric SIV spike contains a primary mass, with two secondary lobes. The transmembrane glycoprotein ‘stalk’ of each trimer is composed of three independent legs that project obliquely from the trimer head, tripod-like. Reconciling available atomic structures with the three-dimensional whole spike density map yields insights into the orientation of Env spike structural elements and possible structural bases of their functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003)
Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl Acad. Sci. USA 100, 4144–4149 (2003)
Poignard, P., Saphire, E. O., Parren, P. & Burton, D. Gp120: biologic aspects of structural features. Annu. Rev. Immunol. 19, 253–274 (2001)
Parren, P. W. & Burton, D. R. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77, 195–262 (2001)
Burton, D. R. et al. HIV vaccine design and the neutralizing antibody problem. Nature Immunol. 5, 233–236 (2004)
Mascola, J. R. et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73, 4009–4018 (1999)
Chan, D. C., Fass, D., Berger, J. M. & Kim, P. S. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 (1997)
Weissenhorn, W., Dessen, A., Harrison, S. C., Skehel, J. J. & Wiley, D. C. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387, 426–430 (1997)
Caffrey, M. et al. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 17, 4572–4584 (1998)
Doms, R. W. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276, 229–237 (2000)
Kwong, P. D. et al. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659 (1998)
Kwong, P. D., Wyatt, R., Sattentau, Q. J., Sodroski, J. & Hendrickson, W. A. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 74, 1961–1972 (2000)
Chen, B. et al. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433, 834–841 (2005)
Zhu, P. et al. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl Acad. Sci. USA 100, 15812–15817 (2003)
Lucic, V., Forster, F. & Baumeister, W. Structural studies by electron tomography: from cells to molecules. Annu. Rev. Biochem. 74, 833–865 (2005)
LaBranche, C. C. et al. A single acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 69, 5217–5227 (1995)
Kodama, T. et al. Significance of premature stop codons in env of simian immunodeficiency virus. J. Virol. 63, 4709–4714 (1989)
Yuste, E., Reeves, J. D., Doms, R. W. & Desrosiers, R. C. Modulation of Env content in virions of simian immunodeficiency virus: correlation with cell surface expression and virion infectivity. J. Virol. 78, 6775–6785 (2004)
Doms, R. W. & Moore, J. P. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151, F9–F13 (2000)
Kuhmann, S. E., Platt, E. J., Kozak, S. L. & Kabat, D. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74, 7005–7015 (2000)
Cavacini, L. A., Emes, C. L., Power, J., Duval, M. & Posner, M. R. Effect of antibody valency on interaction with cell-surface expressed HIV-1 and viral neutralization. J. Immunol. 152, 2538–2545 (1994)
Klasse, P. J. & Sattentau, Q. J. Mechanisms of virus neutralization by antibody. Curr. Top. Microbiol. Immunol. 260, 87–108 (2001)
Yuste, E., Johnson, W., Pavlakis, G. N. & Desrosiers, R. C. Virion envelope content, infectivity, and neutralization sensitivity of simian immunodeficiency virus. J. Virol. 79, 12455–12463 (2005)
Forster, M. J., Mulloy, B. & Nermut, M. V. Molecular modelling study of HIV p17gag (MA) protein shell utilising data from electron microscopy and X-ray crystallography. J. Mol. Biol. 298, 841–857 (2000)
Hill, C. P., Worthylake, D., Bancroft, D. P. & Christensen, A. M. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: Implications for membrane association and assembly. Proc. Natl Acad. Sci. USA 93, 3099–3104 (1996)
Förster, F., Medalia, O., Zauberman, N., Baumeister, W. & Fass, D. Retrovirus envelope protein complex structure in situ studied by cryo-electron tomography. Proc. Natl Acad. Sci. USA 102, 4729–4734 (2005)
Zwick, M. B. et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75, 10892–10905 (2001)
Huang, C. et al. Structure of a V3-containing HIV-1 gp120 core. Science 310, 1025–1028 (2005)
Tan, K., Liu, J.-H., Wang, J.-H., Shen, S. & Lu, M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl Acad. Sci. USA 94, 12303–12308 (1997)
Qiao, Z. et al. Design, expression, and immunogenicity of a soluble HIV trimeric envelope fragment adopting a prefusion gp41 configuration. J. Biol. Chem. 280, 23138–23146 (2005)
Salzwedel, K., West, J. T. & Hunter, E. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73, 2469–2480 (1999)
Saez-Cirion, A. et al. Structural and functional roles of HIV-1 gp41 pretransmembrane sequence segmentation. Biophys. J. 85, 3769–3780 (2003)
Grundner, C., Mirzabekov, T., Sodroski, J. & Wyatt, R. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J. Virol. 76, 3511–3521 (2002)
Ofek, G. et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78, 10724–10737 (2004)
Cardoso, R. M. et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22, 163–173 (2005)
Yuste, E. et al. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV-1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 80, 3030–3041 (2006)
Binley, J. M. et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78, 13232–13252 (2004)
West, J. T., Johnston, P. B., Dubay, S. R. & Hunter, E. Mutations within the putative membrane-spanning domain of the simian immunodeficiency virus transmembrane glycoprotein define the minimal requirements for fusion, incorporation, and infectivity. J. Virol. 75, 9601–9612 (2001)
Yang, X., Kurteva, S., Ren, X., Lee, S. & Sodroski, J. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J. Virol. 79, 12132–12147 (2005)
Blumenthal, R., Sarkar, D. P., Durell, S., Howard, D. E. & Morris, S. J. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J. Cell Biol. 135, 63–71 (1996)
Danieli, T., Pelletier, S. L., Henis, Y. I. & White, J. M. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J. Cell Biol. 133, 559–569 (1996)
Freed, E. O. & Martin, M. A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70, 341–351 (1996)
Rao, Z. et al. Crystal structure of SIV matrix antigen and implications for virus assembly. Nature 378, 743–747 (1995)
Moore, P. L. et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80, 2515–2528 (2006)
Chertova, E. et al. Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins. Curr. Mol. Med. 3, 265–272 (2003)
Rossio, J. L. et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72, 7992–8001 (1998)
Winkler, H. & Taylor, K. A. Accurate marker-free alignment with simultaneous geometry determination and reconstruction of tilt series in electron tomography. Ultramicroscopy 106, 240–254 (2006)
Winkler, H. & Taylor, K. A. Multivariate statistical analysis of three-dimensional cross-bridge motifs in insect flight muscle. Ultramicroscopy 77, 141–152 (1999)
Acknowledgements
We thank H. Winkler for assistance with volume alignment and classification and 3D volume pickup and P. D. Kwong for his comments on the manuscript. The work was supported in part by a NIH and NCI contract (J.B., E.C., J.D.L.) and grants from the NIH NIAID (P.Z., H.G., K.H.R.) and the NIH NIGMS (J.L., K.A.T.). This project has been funded in whole or in part with federal funds from the National Cancer Institute, NIH. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.Author Contributions P.Z. performed the bulk of the cryoEM, image analysis, model fitting, and graphic illustration. J.L. assisted with the image acquisition, analysis and interpretation. J.B. cultured and purified the viruses. E.C. biochemically analysed the virus preparations for purity. J.D.L. contributed to the design of the project and contributed extensively to the manuscript writing. H.G. assisted with fitting of the 2F5 and 4E10 peptides and MAbs into the cryoEM density map. G.A.O. contributed to the analysis of the data, particularly as it relates to the transmembrane region of the envelope spike. K.A.T. provided technical expertise on various aspects of the cryoEM and tomography and contributed to technical portions of the manuscript. K.H.R. provided overall management of the project as well as data interpretation, manuscript writing and figure design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods, Supplementary Figure Legends, and Supplementary Movie Legends and additional references. (DOC 46 kb)
Supplementary Figure 1
Distribution of inter-spike centre-to-centre distances for mutant SIV and wtHIV-1. (JPG 78 kb)
Supplementary Figure 2
Distribution of Env spike cluster sizes on 40 HIV-1 virions compared with the distribution of clusters on hypothetical model virions with randomly placed Env spikes calculated, as described for Supplementary Figure 1. (JPG 45 kb)
Supplementary Figure 3
Inherent three-fold symmetry in Env spike averaged images. (JPG 76 kb)
Supplementary Figure 4
Z-stack of 42 sequential density sections through the 3-fold axis of the final Env spike average. (JPG 77 kb)
Supplementary Figure 5
Comparison of gp120 fitted trimer models. (JPG 148 kb)
Supplementary Figure 6
Density distribution profile of the cryoEM trimer slice through the final Env spike average showing the minimum separation between distinguishable internal density peaks measuring 3.2 nm. (JPG 44 kb)
Supplementary Movie 1
CryoEM tomogram of SIV virions and corresponding isosurface model. (MOV 9749 kb)
Supplementary Movie 2
CryoEM spike Env model. (MOV 9115 kb)
Supplementary Movie 3
De novo fitting of gp120 atomic model into cryoEM isosurface model. (MOV 9282 kb)
Supplementary Movie 4
CryoEM isosurface model manually fitted with gp41 peptides and docked with MAbs. (MOV 8508 kb)
Rights and permissions
About this article
Cite this article
Zhu, P., Liu, J., Bess, J. et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441, 847–852 (2006). https://doi.org/10.1038/nature04817
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nature04817
This article is cited by
-
Inadequate structural constraint on Fab approach rather than paratope elicitation limits HIV-1 MPER vaccine utility
Nature Communications (2023)
-
Phagocytosis by an HIV antibody is associated with reduced viremia irrespective of enhanced complement lysis
Nature Communications (2022)
-
Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival
Nature Communications (2021)
-
Structural basis of transmembrane coupling of the HIV-1 envelope glycoprotein
Nature Communications (2020)
-
Mesoscale computational protocols for the design of highly cooperative bivalent macromolecules
Scientific Reports (2020)