Abstract
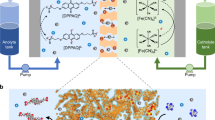
For renewable energy sources such as solar, wind, and hydroelectric to be effectively used in the grid of the future, flexible and scalable energy-storage solutions are necessary to mitigate output fluctuations. Redox-flow batteries (RFBs) were first built in the 1940s and are considered a promising large-scale energy-storage technology. A limited number of redox-active materials—mainly metal salts, corrosive halogens, and low-molar-mass organic compounds—have been investigated as active materials, and only a few membrane materials, such as Nafion, have been considered for RFBs. However, for systems that are intended for both domestic and large-scale use, safety and cost must be taken into account as well as energy density and capacity, particularly regarding long-term access to metal resources, which places limits on the lithium-ion-based and vanadium-based RFB development. Here we describe an affordable, safe, and scalable battery system, which uses organic polymers as the charge-storage material in combination with inexpensive dialysis membranes, which separate the anode and the cathode by the retention of the non-metallic, active (macro-molecular) species, and an aqueous sodium chloride solution as the electrolyte. This water- and polymer-based RFB has an energy density of 10 watt hours per litre, current densities of up to 100 milliamperes per square centimetre, and stable long-term cycling capability. The polymer-based RFB we present uses an environmentally benign sodium chloride solution and cheap, commercially available filter membranes instead of highly corrosive acid electrolytes and expensive membrane materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janoschka, T., Martin, N., Martin, U. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 534, S9–S10 (2016). https://doi.org/10.1038/nature18909
Published:
Issue date:
DOI: https://doi.org/10.1038/nature18909