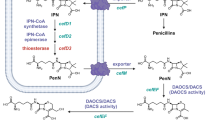
Abstract
A high cephalosporin C producing strain of Cephalosporium acremonium produces higher levels of two enzymes of the bio–synthetic pathway than does a low–producing strain. More importantly, the deacetoxycephalosporin C synthetase of the superior strain appears to be deregulated with respect to carbon source repression. Thus screening or selecting for repression–resistant mutants would appear to be a useful general strategy for obtaining improved antibiotic producers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Demain, A.L. 1983. Biosynthesis of β-lactam antibiotics, p. 189–228. In: Antibiotics Containing the β-lactani Structure. Demain, A. L. and Solomon, N. A. (eds.), Springer-Verlag, Heidelberg.
Wolfe, S., Demain, A.L., Jensen, S.E., and Westlake, D.W.S. 1984. Enzymatic approach to synthesis of unnatural beta-lactams. Science 226:1386–1392.
Shen, Y.-Q., Wolfe, S., and Demain, A.L. 1984. Desacetoxycephalosporin C synthetase: importance of order of cofactor/reactant addition. Enzyme Microb. Technol. 6:402–404.
Heim, J., Shen, Y.-Q., Wolfe, S., and Demain, A.L. 1984. Regulation of isopenicillin N synthetase and deacetoxycephalosporin C synthetase by carbon source during the fermentation of Cephalosporium acremonium. Appl. Microbiol. Biotechnol. 19:232–236.
Shen, Y.-Q., Heim, J., Solomon, N.A., Wolfe, S., and Demain, A.L. 1984. Repression of β-lactam production in Cephalosporium acremonium by nitrogen sources. J. Antibiot. 37:503–511.
Demain, A.L. 1983. Strain exchange between industry and academia. ASM News 49:431.
Hollander, I.J., Shen, Y.-Q., Heim, J., Demain, A.L., and Wolfe, S. 1984. A pure enzyme catalyzing penicillin biosynthesis. Science 224:610–612.
Drew, S.W. and Demain, A.L. 1975. The obligatory role of methionine in the conversion of sulfate to cephalosporin C. Eur. J. Appl. Microbiol. 1:121–128.
Park, J.T. and Johnson, M.J. 1949. A submicrodetermination of glucose. J. Biol. Chem. 181:149–151.
Bradford, M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye binding. Analyt. Biochem. 12:248–254.
Kupka, J., Shen, Y.-Q., Wolfe, S., and Demain, A.L. 1983. Studies on the ring-cyclization and ring-expansion enzymes of β-lactam biosynthesis in Cephalosporium acremonium. Can. J. Microbiol. 29:488–496.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shen, YQ., Wolfe, S. & Demain, A. Levels of Isopenicillin N Synthetase and Deacetoxycephalosporin C Synthetase in Cephalosporium acremonium Producing High and Low Levels of Cephalosporin C. Nat Biotechnol 4, 61–64 (1986). https://doi.org/10.1038/nbt0186-61
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nbt0186-61
This article is cited by
-
Transcriptome analysis of the two unrelated fungal β-lactam producers Acremonium chrysogenum and Penicillium chrysogenum: Velvet-regulated genes are major targets during conventional strain improvement programs
BMC Genomics (2017)
-
Expression of cefF significantly decreased deacetoxycephalosporin C formation during cephalosporin C production in Acremonium chrysogenum
Journal of Industrial Microbiology and Biotechnology (2012)
-
Expression of cefD2 and the conversion of isopenicillin N into penicillin N by the two-component epimerase system are rate-limiting steps in cephalosporin biosynthesis
Molecular Genetics and Genomics (2004)