Abstract
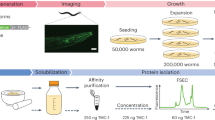
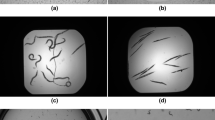
We describe conditions for reproducible large scale cultivation of the free living nematode, Caenorhabditis elegans, and downstream processing of large quantities of this tissue. We were able to grow and harvest 500 grams of purified worms from a 150–liter bioreactor. Cultivation on this scale was accomplished in three steps by using worms from 50 agar plates as inoculum for 4 liters of liquid media. Mature worms from the 4–liter culture were transferred to 20 liters of fresh liquid media. After three days of incubation, the 20–liter culture was used for inoculation of the 150–liter tank. At the time of harvest, a mixture of developmental stages of worms was present. Healthy, motile, egg–laden adults worms appeared to make up the major portion of the population. Membranes prepared from sucrose purified worms were biologically active in an ivermectin binding assay. The affinity of ivermectin (Kd) and the density of receptors (Bmax) were comparable to values generated using membranes from worms grown on agar plates. The amount of membrane tissue obtained from the 500 gm worm harvest made it feasible to consider purification to homogeneity of a nematode protein such as the ivermectin receptor, which is not abundant in crude homogenates. In addition, RNA isolated from sucrose purified worms was translated in Xenopus oocytes. Several channel proteins expressed from this RNA have been characterized, including a chloride channel sensitive to ivermectin and glutamate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sulston, J. and Hodgkin, J. 1988. Methods, p. 587–606. In: The Nematode Caenorhabditis elegans. Wood, W.B. (Ed.). Cold Spring Harbor Laboratory Press, New York.
Chalfie, M. and White, J. 1988. The nervous system, p. 337–391. Ibid.
Horvitz, H.R. 1988. Genetics of cell lineage, p. 157–190. Ibid.
Coulson, A., Sulston, J., Brenner, S. and Karn, J. 1986. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 83: 7821–7825.
Coulson, A., Waterson, R., Kiff, J., Sulston, J. and Kohara, Y. 1988. Genome linking with yeast artificial chromosomes. Nature 335: 184–186.
Watson, J.D. 1990. The human genome project: past, present and future. Science 248: 44–48.
Lewin, R. 1990. A worm at the heart of the genome project. New Scientist August 25: 38–42.
Wood, W.B. 1988. Introduction to C. elegans biology, p. 1–16. In: The Nematode Caenorhabditis elegans. Wood, W.B. (Ed.). Cold Spring Harbor Laboratory Press, NY.
Schaeffer, J.M. and Haines, H.W. 1989. Avermectin binding in Caenorhabditis elegans: A two-state model for the avermectin binding site. Biochem. Pharm. 38: 2329–2338.
Schaeffer, J.M. and Bergstorm, A.R. 1988. Identification of gamma-amino-butyric acid and its binding sites in Caenorhabditis elegans. Life Sci. 43: 1701–1706.
Schaeffer, J.M., White, T., Bergstorm, A.R., Wilson, K.E. and Turner, M. J. 1990. Pesticide Biochem. and Physiol. 36: 220–228.
Vanfleteran, J.R. 1976. Large scale cultivation of a free-living nematode. Experientia 32: 1087–1088.
Salih, N.E. and Hasan, S.A. 1987. Effect of pH of the medium on growth and reproduction of Caenorhabditis (Nematodia: Rhabditidae). J. of Biol. Sci. Research 18(1): 137–143.
Cully, D.F. and Paress, P.S. 1991. Solubilization and characterization of a high affinity Ivermectin binding site from Caenorhabditis elegans. Molecular Pharmacology 40: 326–336.
Rohrer, S.P., Meinke, P.T., Hayes, E.C., Mrozik, H. and Schaeffer, J.M. 1992. Photoaffinity labeling of avermectin binding sites from Caenorhabditis elegans and Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 89: 4168–4172.
Arena, J.P., Liu, K.K., Paress, P.S. and Cully, D.F. 1991. Avermectinsensitive chloride currents induced by Caenorhabditis elegans RNA in Xenopus oocytes. Molecular Pharmacology 40: 368–374.
Arena, J.P., Liu, K.K., Paress, P.S., Schaeffer, J.M. and Cully, D.F. 1992. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Molecular Brain Research 15: 339–348.
Buckland, B., Brix, T., Fastert, H., Gbewonyo, K., Hunt, G. and Jain, D. 1985. Fermentation exhaust gas analysis using mass spectrometry. Bio/Technology 3: 982–988.
Brix, T., Drew, S.W. and Buckland, B.C. 1988. Fermentation process development within a computer controlled pilot plant, p. 151–193. In: Computers in Fermentation Technology. Bushell, M.E. (Ed.). Elsevier Science, Amsterdam.
Lu, N.C., Cheng, A.C. and Briggs, G.M. 1983. A study of mineral requirements of Caenorhabditis elegans. Nematologia 29: 425–434.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gbewonyo, K., Rohrer, S., Lister, L. et al. Large Scale Cultivation of the Free Living Nematode Caenorhabditis elegans. Nat Biotechnol 12, 51–54 (1994). https://doi.org/10.1038/nbt0194-51
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nbt0194-51
This article is cited by
-
The gastrula transition reorganizes replication-origin selection in Caenorhabditis elegans
Nature Structural & Molecular Biology (2017)