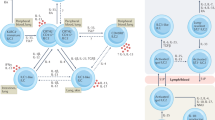
Abstract
Interleukin 2 (IL-2), a T lymphocytes product released upon antigen stimulation, has been used for cancer therapy in high doses for more than five years. More recently, its potential as a stimulant of cell-meidated immunity in infectious diseases, particularly those caused by intracellular microbes, has become appreciated. Drawing on the extensive information available as to the structure, cellular and molecular effects of IL-2, this review focuses on its use in patients with lepromatous leprosy and AIDS in low, physiologic doses. The data indicate that IL-2 is effective in stimulating cell-mediated immunity without systemic toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Smith, K.A. 1988. Interleukin 2: Inception, impact, and implications. Science. 240: 1169–1176.
Smith, K.A. 1989. The interleukin 2 receptor. Annual Review of Cell Biology 5: 397–425.
Brandhuber, B.J., Boone, T. and Kenney, W.C. 1987. Three-dimensional structure of interleukin-2. Science. 238: 1701–1709.
Crabtree, G.R. 1989. Contingent genetic regulatory events in T lymphocyte activation. Science 243: 355–361.
Robb, R.J., Munck, A. and Smith, K.A. 1981. T-cell growth factor receptors: Quantitation, specificity and biological relevance. J. Exp. Med. 154: 1455–1474.
Wang, H.-M. and Smith, K.A. 1987. The interleukin 2 receptor: Functional consequences of its bimolecular structure. J. Exp. Med. 166: 1055–1069.
Hatekeyama, M., Tsudo, M., Minamoto, S., Kono, T., Doi, T., Miyata, T., Miyasaka, M. and Taniguchi, T. 1989. Interleukin-2 receptor β chain gene: generation of three receptor forms by cloned human α and β chain cDNAs. Science 252: 1523–1528.
Zmuidzinas, A., Mamon, H.J., Roberts, T.M. and Smith, K.A. 1991. Interleukin-2 triggered raf-1 expression, phosphorylation and associated kinase activity increase through G1 and S in CD3 stimulated primary human T cells. Molec. Cell Biol. 11: 2794–2803.
Hatakeyama, M., Knon, T., Kobayashi, N., Kawahara, A., Levin, S.D., Perlmutter, R.M. and Taniguchi, T. 1991. Interaction of the IL-2 receptor with the src-family kinase p56lck: Identification of novel intermolecular association. Science 252: 1523–1528.
Gaulton, G.N. and Eardley, D.D. 1986. Interleukin 2-dependent phosphorylation of interleukin 2 receptors and other T cell membrane proteins. J. Immunol. 136: 2470–2477.
Saltzman, E.M., Thom, R.R. and Casnellie, J.E. 1988. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J. Biol. Chem. 263: 6956–6959.
Saltzman, E.M., White, K. and Casnelli, J.E. 1990. Stimulation of the antigen and interleukin-2 receptors on T lymphocytes activates distinct tyrosine protein kinases. J. Biol. Chem. 265: 10138–10142.
Asao, H., Takeshita, T., Nakamura, M., Nagata, K. and Sugamura, K. 1990. Interleukin 2 (IL-2)-induced tyrosine phosphorylation of IL-2 receptor p75. J. Exp. Med. 171: 637–644.
Merida, I., Pratt, J.C. and Gaulton, G.G. 1990. Regulation of interleukin 2-dependent growth responses by glycosylphosphatidylinositol molecules. Proc. Natl. Acad. Sci. USA 87: 9421–9425.
Eardley, D.D. and Koshland, M.E. 1991. Glycosylphosphatidylinositol: A candidate system for interleukin-2 signal transductionE. Science. 251: 78–81.
Reed, J.C., Alpers, J.D., Nowell, P.C. and Hoover, R.G. 1986. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc. Natl. Acad. Sci. USA 83: 3982–3986.
Dautry, F., Weil, D., Yu, J. and Dautry-Varsat, A. 1988. Regulation of pim and myb mRNA accumulation by interleukin 2 and interleukin 3 in murine hematopoietic cell lines. J. Biol. Chem. 33: 17615–17620.
Stern, J.B. and Smith, K.A. 1986. Interleukin 2 induction of T cell G1 progression and c-myb expressionA. Science 233: 203–206.
Beadling, C., Johnson, K. and Smith, K.A. In preparation.
Caligiuri, M.A., Zmuidzinas, A., Manley, T.J., Levin, H., Smith, K.A. and Ritz, J. 1990. Functional consequences of IL-2 receptor expression on resting human lymphocytes: Identification of a Novel NK cell subset with high affinity receptors. J. Exp. Med. 171: 1509–1526.
Michael Brenner—personal communication.
Ritz, J., Schmidt, R.E., Michon, J., Hercend, T. and Schlossman, S.F. 1988. Characterization of functional surface structures on human natural killer cells. Advances in Immunology 42: 181–211.
Voss, S.D., Robb, R.J., Weil-Hillman, G., Hank, J.A., Sugamura, K., Tsudo, M. and Sondel, P.M. 1990. Increased expression of the interleukin 2 (IL-2) receptor β chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J. Exp. Med. 172: 1101–1114.
Caligiuri, M., Smith, K.A. and Ritz, J.A. In preparation.
Baume, D., Ehlers, S., Ritz, J.A., Smith, K.A. Unpublished
Liu, C-C., Rafii, S., Granelli-Piperno, A., Trapani, J.A. and Young, J.D.-E. 1989. Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effect of cyclosporin A. J. Exp. Med. 170: 2105–2118.
Biron, C.A., Byron, K.A. and Sullivan, J.L. 1989. Severe herpersvirus infections in an adolescent without natural killer cells. N. Eng. J. Med. 320: 1731–1735.
Molloy, A., Smith, K.D. and Kaplan, G. The selective recognition of BCG-infected monocytes by cytolytic cells and th effect of cytolysis on parasite viability. In preparation.
Blackmen, M.A., Tigges, M.A., Minie, M.E. and Koshland, M.E. 1986. A model system for peptide hormone action in differentiation: Interleukin 2 induces a B lymphoma to transcribe the J chain gene. Cell. 47: 609–617.
Ehlers, S. and Smith, K.A. 1991. Differentiation of T cell lymphokine gene expression: The in vitro acquisition of T cell memory. J. Exp. Med. 173: 25–36.
Kaplan, G., Nusrat, A., Witmer, M.D., Nath, I. and Cohn, Z.A. 1987. Distribution and turnover of Langerhans cells during delayed immune responses in human skin. J. Exp. Med. 165: 763–776.
Kaplan, G., Kiessling, R., Teklemariam, S., Hancock, G., Sheftel, G., Job, C.K., Converse, P., Ottenhoff, T.H.M., Becx-Bleumink, M., Dietz, M. and Cohn, Z.A. 1989. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J. Exp. Med. 169: 893–907.
Kalpan, G., Nusrat, A., Sarno, E.N., Job, C.K., McElrath, J., Porto, J.A., Nathan, C.F. and Cohn, Z.A. 1987. Cellular responses to the intradermal injection of recombinant human γ-interferon in Lepromatous leprosy patients. Am. J. of Path. 128: 345–349.
Kaplan, G., Mathur, N.K., Job, C.K., Nath, I. and Cohn, Z.A. 1989. Effect of multiple interferon γ injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. USA 86: 8073–8077.
Pahwa, R., Chatila, T., Pahwa, S., Paradise, C., Day, N.K., Geha, R., Schwartz, S.A., Slade, H., Oyaizu, N. and Good, R.A. 1989. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc. Natl. Acad. Sci. USA. 86: 5069–5073.
Weinberg, K. and Parkman, R. 1991. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N. Eng. J. Med. 322: 1718–1723.
Schorle, H., Holtschke, T., Hunig, T., Schimpl, A. and Horak, I. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352: 621–624.
Kaplan, G., Britton, W.J., Hancock, G.E., Theuvenet, W.J., Smith, K.A., Job, C.K., Roche, P.W., Molloy, A., Burkhardt, R., Barker, J., Pradhan, H.M. and Cohen, Z.A. 1991. The systemic influence of recombinant interleukin 2 on the manifestations of Lepromatous leprosy. J. Exp. Med. 173: 993–1006.
McElrath, M.J., Kaplan, G., Burkhardt, R.A. and Cohn, Z.A. 1990. Cutaneous response to recombinant interleukin 2 in human immunodeficiency virus 1-seropositive individuals. Proc. Natl. Acad. Sci. USA 87: 5783–5787.
Lotze, M.T., Matory, Y.L., Ettinghausen, S.E., Rayner, A.A., Sharrow, S.O., Seipp, C.A.Y., Custer, M.C. and Rosenberg, S.A. 1985. In vivo administration of purified human interleukin 2 II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 135: 2865–2875.
Mier, J.W., Vachino, G., Klernpner, M.S., Aronson, F.R., Noring, R., Smith, S., Brandon, E.P., Laird, W. and Atkins, M.B. 1990. Inhibition of interleukin-2-induced tumor necrosis factor release by dexamethasone: prevention of an acquired neutrophil chemotaxis defect and differential suppression of interleukin-2-associated side effects. Blood 76: 1933–1940.
Caligiuri, M.A., Murray, C., Smith, K.A. and Ritz, J. Selective immune modulation following extended in vivo infusion of low does IL-2. In preparation.
Caligiuri, M.A., Murray, C., Soiffer, R.J., Klumpp, T.R., Selden, M., Cochran, K., Cameron, C., Ish, C., Buchanan, L., Perillo, D., Smith, K.A. and Ritz, J. 1991. Extended continuous infusion of low dose recombinant IL-2 in advanced cancer: prolonged immune modulation without significant toxicity. J. Clin. Onc. In press.
Sampaio, E.P., Sarno, E.N., Galilly, R., Cohn, Z.A. and Kaplan, G. 1991. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J. Exp. Med. 173: 699–703.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaplan, G., Cohn, Z. & Smith, K. Rational Immunotherapy with Interleukin 2. Nat Biotechnol 10, 157–162 (1992). https://doi.org/10.1038/nbt0292-157
Issue date:
DOI: https://doi.org/10.1038/nbt0292-157