Abstract
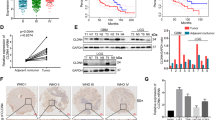
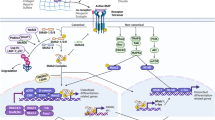
CLIC4 (chloride intracellular channel 4), a multifunctional protein that traffics between the cytoplasm and nucleus, interacts with Schnurri-2, a transcription factor in the bone morphogenetic protein (BMP) signalling pathway. Here we show that transforming growth factor β (TGF-β) promotes the expression of CLIC4 and Schnurri-2 as well as their association in the cytoplasm and their translocation to the nucleus. In the absence of CLIC4 or Schnurri-2, TGF-β signalling is abrogated. Direct nuclear targeting of CLIC4 enhances TGF-β signalling and removes the requirement for Schnurri-2. Nuclear CLIC4 associates with phospho (p)-Smad2 and p-Smad3, protecting them from dephosphorylation by nuclear phosphatases. An intact TGF-β signalling pathway is essential for CLIC4-mediated growth-arrest. These results newly identify Schnurri-2 and CLIC4 as modifiers of TGF-β signalling through their stabilization of p-Smad2 and 3 in the nucleus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Suh, K. S. & Yuspa, S. H. Intracellular chloride channels: critical mediators of cell viability and potential targets for cancer therapy. Curr. Pharm. Des. 11, 2753–2764 (2005).
Littler, D. R. et al. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 279, 9298–9305 (2004).
Littler, D. R. et al. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 272, 4996–5007 (2005).
Shorning, B. Y., Wilson, D. B., Meehan, R. R. & Ashley, R. H. Molecular cloning and developmental expression of two chloride intracellular channel (CLIC) genes in Xenopus laevis. Dev. Genes Evol. 213, 514–518 (2003).
Fernandez-Salas, E. et al. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 22, 3610–3620 (2002).
Shiio, Y. et al. Quantitative proteomic analysis of Myc-induced apoptosis: a direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J. Biol. Chem. 281, 2750–2756 (2006).
Suh, K. S. et al. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J. Biol. Chem. 279, 4632–4641 (2004).
Suh, K. S. et al. CLIC4 mediates and is required for Ca2+-induced keratinocyte differentiation. J. Cell Sci. 120, 2631–2640 (2007).
Suh, K. S. et al. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin. Cancer Res. 13, 121–131 (2007).
Ronnov-Jessen, L., Villadsen, R., Edwards, J. C. & Petersen, O. W. Differential expression of a chloride intracellular channel gene, CLIC4, in transforming growth factor-β1-mediated conversion of fibroblasts to myofibroblasts. Am. J. Pathol. 161, 471–480 (2002).
Dai, H. et al. The zinc finger protein schnurri acts as a Smad partner in mediating the transcriptional response to decapentaplegic. Dev. Biol. 227, 373–387 (2000).
Jin, W. et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev. Cell 10, 461–471 (2006).
Udagawa, Y. et al. Schnurri interacts with Mad in a Dpp-dependent manner. Genes Cells 5, 359–369 (2000).
Liu, X. et al. Conditional epidermal expression of TGFβ 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc. Natl Acad. Sci. USA 98, 9139–9144 (2001).
Pietenpol, J. A. et al. TGF-β1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell 61, 777–785 (1990).
Datto, M. B. et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA 92, 5545–5549 (1995).
Mavrakis, K. J. et al. Arkadia enhances Nodal/TGF-β signaling by coupling phospho-Smad2/3 activity and turnover. PLoS Biol. 5, e67 (2007).
Lin, X. et al. PPM1A functions as a Smad phosphatase to terminate TGFβ signaling. Cell 125, 915–928 (2006).
Hill, C. S. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 19, 36–46 (2009).
Levy, L. et al. Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol. Cell. Biol. 27, 6068–6083 (2007).
Varelas, X. et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biol. 10, 837–848 (2008).
Lichti, U., Anders, J. & Yuspa, S. H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nature Protoc. 3, 799–810 (2008).
Takagi, T., Harada, J. & Ishii, S. Murine Schnurri-2 is required for positive selection of thymocytes. Nature Immunol. 2, 1048–1053 (2001).
Fields, S. & Song, O. A novel genetic system to detect protein–protein interactions. Nature 340, 245–246 (1989).
Fernandez-Salas, E., Sagar, M., Cheng, C., Yuspa, S. H. & Weinberg, W. C. p53 and tumor necrosis factor α regulate the expression of a mitochondrial chloride channel protein. J. Biol. Chem. 274, 36488–36497 (1999).
Acknowledgements
The authors thank L. Wakefield for critically reading the manuscript, M. Anzano for help with genotyping, C. Cheng for help with immunoprecipitation assays and colleagues for providing constructive criticism throughout the study. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
Author information
Authors and Affiliations
Contributions
A.S. designed and carried out studies, analysed data and wrote the manuscript; C.C. performed the analysis of in vivo samples and contributed to writing the manuscript; Y.H. and T.F. performed immunoblotting, immunofluorescence and luciferase assays; M.M. designed the yeast two-hybrid study;, K.S.H. provided essential reagents; S.H.Y. designed experiments, analysed and organized the data and wrote the manuscript. All authors proofread the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 735 kb)
Rights and permissions
About this article
Cite this article
Shukla, A., Malik, M., Cataisson, C. et al. TGF-β signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol 11, 777–784 (2009). https://doi.org/10.1038/ncb1885
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ncb1885
This article is cited by
-
Chloride intracellular channel (CLIC) proteins function as fusogens
Nature Communications (2024)
-
N6-methyladenosine demethylase FTO suppressed prostate cancer progression by maintaining CLIC4 mRNA stability
Cell Death Discovery (2022)
-
Retinal pigment epithelium-specific CLIC4 mutant is a mouse model of dry age-related macular degeneration
Nature Communications (2022)
-
Induction of tolerogenic dendritic cells by activated TGF-β/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells
BMC Cancer (2019)
-
Varroa destructor parasitism has a greater effect on proteome changes than the deformed wing virus and activates TGF-β signaling pathways
Scientific Reports (2019)