Figure 1: HOR on Pt in a full range of solution pH and modelled HOR polarization curves.
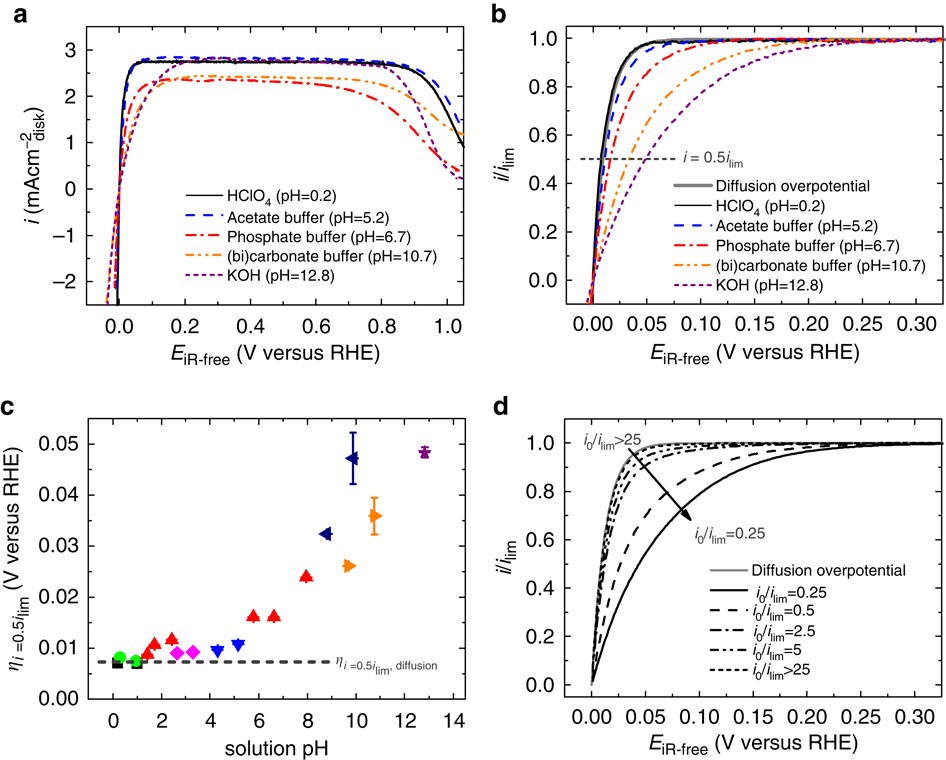
(a) Steady state positive-going sweeps of HOR polarization curves of Pt collected in selected H2-saturated buffer solutions. The sweep rate is 10 mV s−1 and the rotating speed is 1,600 r.p.m. The polarization curves have been corrected for solution resistance. (b) HOR polarization curves normalized to the maximum limiting current density ilim. (c) Half-wave potential of the HOR in all pH-buffered electrolytes. Error bars are s.d. of at least two sets of experimental repeats. Black squares, perchloric acids (0.1 and 1 M); green circles, sulfuric acids (0.1 and 1 M); red up-triangles, phosphoric acid (0.1 M) and phosphate buffers; blue down-triangles, acetate buffers; magenta diamonds, citrate buffers; navy left-triangles, borate buffers; orange right-triangles, (bi)carbonate buffers; and purple stars, potassium hydroxide solution (0.1 M). Identical colour schemes are used for symbols in other figures. (d) Modelled HOR polarization curves using equations (2)–(4), , .
