Abstract
Classical forms of congenital adrenal hyperplasia are caused by a severe deficiency of 21-hydroxylase, an enzyme involved in steroid biosynthesis, which triggers excessive androgen production before birth. Affected females experience virilization both physically and psychologically. Prenatal diagnosis and treatment of congenital adrenal hyperplasia has been implemented for more than 20 years. In utero gene-specific diagnosis is now feasible for fetal cell samples derived from chorionic villi or amniotic cells in culture, and this gene-specific diagnosis guides the treatment of the affected female fetus. Appropriate dexamethasone administration to the at-risk pregnant mother is effective in reducing genital virilization in the fetus, and thus avoids unnecessary genitoplasty in affected females. Current data from large human studies show the benefit and safety of prenatal treatment. Long-term follow-up of the safety of prenatal treatment is currently underway. This practice is a rare example of effective prenatal treatment to prevent a malformation caused by an inborn error of metabolism.
Key Points
-
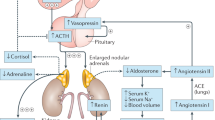
The term congenital adrenal hyperplasia (CAH) applies to a group of disorders affecting the biosynthesis of cortisol; the most common form of CAH is 21-hydroxylase deficiency CAH (21-OHD CAH)
-
In classical (severe or moderately severe) forms of 21-OHD CAH, prenatal androgen excess leads to genital ambiguity in affected females
-
Prenatal diagnosis of 21-OHD CAH is accomplished via genetic analysis of fetal DNA obtained from chorionic villi sampling
-
When appropriately administered, prenatal treatment with dexamethasone is safe and effective in preventing ambiguity of genitalia of female fetuses affected with 21-OHD CAH
-
International follow-up studies of treated mothers and their offspring will confirm the short-term safety and efficacy of prenatal diagnosis and treatment of 21-OHD CAH
-
Studies to determine the long-term outcome of prenatal treatment are indicated and are underway
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
New MI (2004) An update of congenital adrenal hyperplasia. Ann NY Acad Sci 1038: 14–43
New MI (2006) Extensive clinical experience: nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab 91: 4205–4214
White PC and Speiser PW (2000) Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 21: 245–291
Hughes IA et al. (2006) Consensus statement on management of intersex disorders. Arch Dis Child 91: 554–563
Crouch NS and Creighton SM (2004) Minimal surgical intervention in the management of intersex conditions. J Pediatr Endocrinol Metab 17: 1591–1596
Therrell BJ et al. (1998) Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 101: 583–590
Temeck JW et al. (1987) Genetic defects of steroidogenesis in premature pubarche. J Clin Endocrinol Metab 64: 609–617
Pang S et al. (1985) Pitfalls of prenatal diagnosis of 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab 61: 89–97
Gruneiro-Papendieck L et al. (2001) Neonatal screening program for congenital adrenal hyperplasia: adjustments to the recall protocol. Horm Res 55: 271–277
Allen DB et al. (1997) Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J Pediatr 130: 128–133
New MI et al. (1983) Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab 57: 320–326
Nebert DW et al. (1991) The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol 10: 1–14
Dupont B et al. (1977) Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet 2: 1309–1312
White PC et al. (1986) Structure of the human steroid 21-hydroxylase genes. Proc Natl Acad Sci USA 83: 5111–5115
Stenson PD et al. (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21: 577–581
Speiser PW et al. (1992) Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 90: 584–595
Wilson RC et al. (1995) Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab 80: 2322–2329
Villee DB (1972) The development of steroidogenesis. Am J Med 53: 533–544
Goto M et al. (2006) In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116: 953–960
Jeffcoate T et al. (1965) Diagnosis of the adrenogenital syndrome before birth. Lancet 2: 553–555
Mercado AB et al. (1995) Prenatal treatment and diagnosis of congenital adrenal hyperplasia owing to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab 80: 2014–2020
Wilson RC et al. (1995) Rapid deoxyribonucleic acid analysis by allele-specific polymerase chain reaction for detection of mutations in the steroid 21-hydroxylase gene. J Clin Endocrinol Metab 80: 1635–1640
Tukel T et al. (2003) A novel semiquantitative polymerase chain reaction/enzyme digestion-based method for detection of large scale deletions/conversions of the CYP21 gene and mutation screening in Turkish families with 21-hydroxylase deficiency. J Clin Endocrinol Metab 88: 5893–5897
Lee HH et al. (2004) Use of PCR-based amplification analysis as a substitute for the Southern blot method for CYP21 deletion detection in congenital adrenal hyperplasia. Clin Chem 50: 1074–1076
Nimkarn S et al. (1999) Congenital adrenal hyperplasia (21-hydroxylase deficiency) without demonstrable genetic mutations. J Clin Endocrinol Metab 84: 378–381
Day DJ et al. (1996) Identification of non-amplifying CYP21 genes when using PCR-based diagnosis of 21-hydroxylase deficiency in congenital adrenal hyperplasia (CAH) affected pedigrees. Hum Mol Genet 5: 2039–2048
Mao R et al. (2002) Prenatal diagnosis of 21-hydroxylase deficiency caused by gene conversion and rearrangements: pitfalls and molecular diagnostic solutions. Prenat Diagn 22: 1171–1176
Grumbach MM and Shaw EB (1998) Further studies on the treatment of congenital adrenal hyperplasia with cortisone. IV: effect of cortisone and compound B in infants with disturbed electrolyte metabolism, by John F. Crigler Jr, MD, Samuel H. Silverman, MD, and Lawson Wilkins, MD, Pediatrics, 1952; 10: 397–413. Pediatrics 102: 215–221
David M and Forest MG (1984) Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 105: 799–803
Forest M et al. (1989) Prenatal treatment in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: up-date 88 of the French multicentric study. Endocr Res 15: 277–301
New MI et al. (2001) Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 86: 5651–5657
Saenger P (1984) Abnormal sex differentiation. J Pediatr 104: 1–17
Meyer-Bahlburg HF et al. (2006) Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Arch Sex Behav 35: 667–684
Seckl JR (2004) Prenatal glucocorticoids and long-term programming. Eur J Endocrinol 151 (Suppl 3): SU49–SU62
Seckl JR and Miller WL (1997) How safe is long-term prenatal glucocorticoid treatment? JAMA 277: 1077–1079
New MI et al. (2003) Update: prenatal diagnosis for congenital adrenal hyperplasia in 595 pregnancies. Endocrinologist 13: 233–239
Forest MG and Dorr HG (2003) Prenatal therapy in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: retrospective follow-up study of 253 treated pregnancies in 215 families. Endocrinologist 13: 252–259
Trautman PD et al. (1996) Mothers' reactions to prenatal diagnostic procedures and dexamethasone treatment of CAH. J Psychosom Obstet Gynaecol 17: 175–181
Lajic S et al. (1998) Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 83: 3872–3880
Lajic S et al. (2004) Prenatal treatment of congenital adrenal hyperplasia. Eur J Endocrinol 151 (Suppl 3): SU63–SU69
Goldman A et al. (1978) Human foetal palatal corticoid receptors and teratogens for cleft palate. Nature 272: 464–466
Raman PB et al. (1964) Conversion of progesterone-4-14c to 18-hydroxycorticosterone and aldosterone by mouse adrenals in vitro. Endocrinology 74: 865–869
Nandi J et al. (1967) In vitro steroidogenesis by the adrenal glands of mice. Endocrinology 80: 576–582
Yeh TF et al. (2004) Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 350: 1304–1313
Slotkin TA et al. (1998) Glucocorticoid administration alters nuclear transcription factors in fetal rat brain: implications for the use of antenatal steroids. Brain Res Dev Brain Res 111: 11–24
Uno H et al. (1990) Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I: hippocampus. Brain Res Dev Brain Res 53: 157–167
Joint LWPES/ESPE CAH Working Group (2002) Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 87: 4048–4053
Newnham JP (2001) Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol 28: 957–961
Trautman PD et al. (1995) Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology 20: 439–449
Hall C et al. (2004) Behavioral and physical masculinization are related to genotype in girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab 89: 419–424
Pang SY et al. (1990) Prenatal treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med 322: 111–115
Speiser P (1999) Prenatal treatment of congenital adrenal hyperplasia. J Urol 162: 534–536
Rijnders RJ et al. (2001) Fetal sex determination from maternal plasma in pregnancies at risk for congenital adrenal hyperplasia. Obstet Gynecol 98: 374–378
Morel Y et al. (2003) 21 hydroxylase deficiency: new strategies emerging from molecular studies [French]. Ann Endocrinol (Paris) 64: 456–470
Tusie-Luna MT et al. (1991) A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol 5: 685–692
Speiser PW et al. (1988) Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med 319: 19–23
Helmberg A et al. (1992) R339H and P453S: CYP21 mutations associated with nonclassic steroid 21-hydroxylase deficiency that are not apparent gene conversions. Mol Endocrinol 6: 1318–1322
Owerbach D et al. (1992) Pro-453 to ser mutation in CYP21 is associated with nonclassic steroid 21-hydroxylase deficiency. Mol Endocrinol 6: 1211–1215
White PC et al. (1984) HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA 81: 7505–7509
Higashi Y et al. (1988) Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci USA 85: 7486–7490
White PC et al. (1994) Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat 3: 373–378
Amor M et al. (1988) Mutation in the CYP21B gene (Ile-172-Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA 85: 1600–1607
Tusie-Luna M et al. (1990) Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem 265: 20916–20922
Globerman H et al. (1988) Nonsense mutation causing steroid 21-hydroxylase deficiency. J Clin Invest 82: 139–144
Chiou SH et al. (1990) A missense mutation at Ile172—Asn or Arg356—Trp causes steroid 21-hydroxylase deficiency. J Biol Chem 265: 3549–3552
Wedell A and Luthman H (1993) Steroid 21-hydroxylase (P450c21): a new allele and spread of mutations through the pseudogene. Hum Genet 91: 236–240
Nimkarn S and New MI (2006) Prenatal diagnosis and treatment of congenital adrenal hyperplasia. Horm Res 67: 53–60
Acknowledgements
This work is supported in part by US Public Health Service grant HD00072. The authors would like to thank Naomi Horowitz for her editorial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Nimkarn, S., New, M. Prenatal diagnosis and treatment of congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Nat Rev Endocrinol 3, 405–413 (2007). https://doi.org/10.1038/ncpendmet0481
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/ncpendmet0481
This article is cited by
-
Noninvasive prenatal diagnosis of 21-Hydroxylase deficiency using target capture sequencing of maternal plasma DNA
Scientific Reports (2017)
-
High frequency of splice site mutation in 21-hydroxylase deficiency children
Journal of Endocrinological Investigation (2015)
-
Adrenal disorders in pregnancy
Nature Reviews Endocrinology (2012)
-
Dexamethasone normalizes aberrant elastic fiber production and collagen 1 secretion by Loeys–Dietz syndrome fibroblasts: a possible treatment?
European Journal of Human Genetics (2011)
-
Treating fetal thyroid and adrenal disorders through the mother
Nature Clinical Practice Endocrinology & Metabolism (2008)