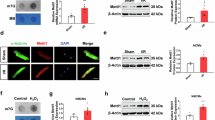
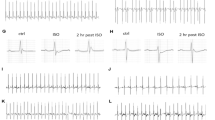
Abstract
Arrhythmogenic right ventricular dysplasia (ARVD) is a hereditary cardiomyopathy that causes sudden death in the young. We found a line of mice with inherited right ventricular dysplasia (RVD) caused by a mutation of the gene laminin receptor 1 (Lamr1). This locus contained an intron-processed retroposon that was transcribed in the mice with RVD. Introduction of a mutated Lamr1 gene into normal mice by breeding or by direct injection caused susceptibility to RVD, which was similar to that seen in the RVD mice. An in vitro study of cardiomyocytes expressing the product of mutated Lamr1 showed early cell death accompanied by alteration of the chromatin architecture. We found that heterochromatin protein 1 (HP1) bound specifically to mutant LAMR1. HP1 is a dynamic regulator of heterochromatin sites, suggesting that mutant LAMR1 impairs a crucial process of transcriptional regulation. Indeed, mutant LAMR1 caused specific changes to gene expression in cardiomyocytes, as detected by gene chip analysis. Thus, we concluded that products of the Lamr1 retroposon interact with HP1 to cause degeneration of cardiomyocytes. This mechanism may also contribute to the etiology of human ARVD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Thiene, G. et al. Arrythmogenic right ventricular cardiomyopathy. Trends Cardiovasc. Med. 7, 84–90 (1997).
Severini, G.M. et al. A new locus for arrhythmogenic right ventricular dysplasia on the long arm of chromosome 14. Genomics 31, 193–200 (1996).
Rampazzo, A. et al. ARVD4, a new locus for arrhythmogenic right ventricular cardiomyopathy, maps to chromosome 2 long arm. Genomics 45, 259–263 (1997).
Ahmad, F. et al. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation 98, 2791–2795 (1998).
Li, D. et al. The locus of a novel gene responsible for arrhythmogenic right- ventricular dysplasia characterized by early onset and high penetrance maps to chromosome 10p12-p14. Am. J. Hum. Genet. 66, 148–156 (2000).
Tiso, N. et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum. Mol. Genet. 10, 189–194 (2001).
Bright, J.M. & McEntee, M. Isolated right ventricular cardiomyopathy in a dog. J. Am. Vet. Med. Assoc. 207, 64–66 (1995).
Simpson, K.W., Bonagura, J.D. & Eaton, K.A. Right ventricular cardiomyopathy in a dog. J. Vet. Intern. Med. 8, 306–309 (1994).
Fox, P.R., Maron, B.J., Basso, C., Liu, S.K. & Thiene, G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: A new animal model similar to the human disease. Circulation 102, 1863–1870 (2000).
Ishikawa, S., Zu Rhein, G.M. & Gilbert, E.F. Uhl's anomaly in the mink. Partial absence of the right atrial and ventricular myocardium. Arch. Pathol. Lab. Med. 101, 388–390 (1977).
Sato, M. et al. Analysis of nuclear localization of laminin binding protein precursor p40 (LBP/p40). Biochem. Biophys. Res. Commun. 229, 896–901 (1996).
Kaneda, Y. et al. The induction of apoptosis in HeLa cells by the loss of LBP-p40. Cell Death Differ. 5, 20–28 (1998).
Kondo, K., Nozawa, K., Tomita, T. & Ezaki, K. Inbred strains resulting from Japanese mice. Bull. Exp. Anim. 6, 107–112 (1967).
Nakamura, M. & Yamada, K. Studies on a diabetic (KK) strain of the mouse. Diabetologia 3, 212–221 (1967).
McKenna, W.J. et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 71, 215–218 (1994).
Lin, H., Parmacek, M.S., Morle, G., Bolling, S. & Leiden, J.M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation 82, 2217–2221 (1990).
Nakayama, J., Rice, J.C., Strahl, B.D., Allis, C.D. & Grewal, S.I. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 (2001).
Festenstein, R. et al. Modulation of heterochromatin protein 1 dynamics in primary Mammalian cells. Science 299, 719–721 (2003).
Cranefield, P. The Conduction of the Cardiac Impulse (Futura, Mount Kisco, New York, 1975).
Peters, S. & Trummel, M. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard ECG revisited. Ann. Noninvasive Electrocardiol. 8, 238–245 (2003).
Nasir, K. et al. Filtered QRS duration on signal-averaged electrocardiography predicts inducibility of ventricular tachycardia in arrhythmogenic right ventricle dysplasia. Pacing Clin. Electrophysiol. 26, 1955–1960 (2003).
Burke, A.P., Farb, A., Tashko, G. & Virmani, R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation 97, 1571–1580 (1998).
Protonotarios, N. et al. Genotype-phenotype assessment in autosomal recessive arrhythmogenic right ventricular cardiomyopathy (Naxos disease) caused by a deletion in plakoglobin. J. Am. Coll. Cardiol. 38, 1477–1484 (2001).
Steenman, M. et al. Transcriptomal analysis of failing and nonfailing human hearts. Physiol. Genomics 12, 97–112 (2003).
Pashmforoush, M. et al. Adult mice deficient in actinin-associated LIM-domain protein reveal a developmental pathway for right ventricular cardiomyopathy. Nat. Med. 7, 591–597 (2001).
Cheutin, T. et al. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299, 721–725 (2003).
Zhang, C.L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Zhang, C.L., McKinsey, T.A. & Olson, E.N. Association of class II histone deacetylases with heterochromatin protein 1: potential role for histone methylation in control of muscle differentiation. Mol. Cell. Biol. 22, 7302–7312 (2002).
McCarrey, J.R. & Thomas, K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326, 501–505 (1987).
Gebara, M.M. & McCarrey, J.R. Protein-DNA interactions associated with the onset of testis-specific expression of the mammalian Pgk-2 gene. Mol. Cell. Biol. 12, 1422–1431 (1992).
Dahl, H.H., Brown, R.M., Hutchison, W.M., Maragos, C. & Brown, G.K. A testis-specific form of the human pyruvate dehydrogenase E1 α subunit is coded for by an intronless gene on chromosome 4. Genomics 8, 225–232 (1990).
Hirotsune, S. et al. An expressed pseudogene regulates the messenger-RNA stability of its homologous coding gene. Nature 423, 91–96 (2003).
Richardson, M.P., Braybrook, C., Tham, M., Moore, G.E. & Stanier, P. Molecular cloning and characterization of a highly conserved human 67- kDa laminin receptor pseudogene mapping to Xq21.3. Gene 206, 145–150 (1998).
Gordon, J.W., Scangos, G.A., Plotkin, D.J., Barbosa, J.A. & Ruddle, F.H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. USA 77, 7380–7384 (1980).
Simpson, P., McGrath, A. & Savion, S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ. Res. 51, 787–801 (1982).
Lockhart, D.J. et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol. 14, 1675–1680 (1996).
Acknowledgements
These data were generated through the use of the Celera Discovery System and Celera Genomics' Associated Databases. We thank T. Tanaka and K. Miyake for assistance with DNA sequencing; S. Hirota for histologic expertise; H. Niwa for donating plasmid constructs and providing suggestions; K. Yamamoto for amino-acid sequencing; and H. Kikutani, K. Node and T. Nakagawa for discussions. This work was supported by Grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health and Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Asano, Y., Takashima, S., Asakura, M. et al. Lamr1 functional retroposon causes right ventricular dysplasia in mice. Nat Genet 36, 123–130 (2004). https://doi.org/10.1038/ng1294
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ng1294
This article is cited by
-
Arrhythmogenic Cardiomyopathy: from Preclinical Models to Genotype–phenotype Correlation and Pathophysiology
Stem Cell Reviews and Reports (2023)
-
The histone 3 lysine 9 methyltransferase inhibitor chaetocin improves prognosis in a rat model of high salt diet-induced heart failure
Scientific Reports (2017)
-
Animal models of arrhythmogenic right ventricular cardiomyopathy: what have we learned and where do we go? Insight for therapeutics
Basic Research in Cardiology (2017)
-
Characterization of the ovine ribosomal protein SA gene and its pseudogenes
BMC Genomics (2010)
-
Structural and functional analysis of the ovine laminin receptor gene (RPSA): Possible involvement of the LRP/LR protein in scrapie response
Mammalian Genome (2008)