Abstract
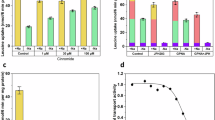
Hartnup disorder (OMIM 234500) is an autosomal recessive abnormality of renal and gastrointestinal neutral amino acid transport noted for its clinical variability. We localized a gene causing Hartnup disorder to chromosome 5p15.33 and cloned a new gene, SLC6A19, in this region. SLC6A19 is a sodium-dependent and chloride-independent neutral amino acid transporter, expressed predominately in kidney and intestine, with properties of system B0. We identified six mutations in SLC6A19 that cosegregated with disease in the predicted recessive manner, with most affected individuals being compound heterozygotes. The disease-causing mutations that we tested reduced neutral amino acid transport function in vitro. Population frequencies for the most common mutated SLC6A19 alleles are 0.007 for 517G → A and 0.001 for 718C → T. Our findings indicate that SLC6A19 is the long-sought gene that is mutated in Hartnup disorder; its identification provides the opportunity to examine the inconsistent multisystemic features of this disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baron, D.N., Dent, C.E., Harris, H., Hart, E.W. & Jepson, J.B. Hereditary pellagra-like skin rash with temporary cerebellar ataxia. Constant renal amino-aciduria. And other bizarre biochemical features. Lancet 2, 421 (1956).
Scriver, C.R. Hartnup Disease: A Genetic Modification of intestinal and renal transport of certain neutral alpha-amino acids. N. Engl. J. Med. 273, 530–532 (1965).
Levy, H.L. Hartnup disorder. in The Metabolic and Molecular Bases of Inherited Disease (eds. Scriver, C.R. et al.) (4957–4969 McGraw Hill, New York, 2001).
Stevens, B.R., Ross, H.J. & Wright, E.M. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J. Membr. Biol. 66, 213–225 (1982).
Munck, L.K. & Munck, B.G. Amino acid transport in the small intestine. Physiol. Res. 43, 335–345 (1994).
Doyle, F.A. & McGivan, J.D. The bovine renal epithelial cell line NBL-1 expresses a broad specificity Na(+)-dependent neutral amino acid transport system (System Bo) similar to that in bovine renal brush border membrane vesicles. Biochim. Biophys. Acta 1104, 55–62 (1992).
Souba, W.W., Pan, M. & Stevens, B.R. Kinetics of the sodium-dependent glutamine transporter in human intestinal cell confluent monolayers. Biochem. Biophys. Res. Commun. 188, 746–753 (1992).
Mailliard, M.E., Stevens, B.R. & Mann, G.E. Amino acid transport by small intestinal, hepatic, and pancreatic epithelia. Gastroenterology 108, 888–910 (1995).
Potter, S.J., Lu, A., Wilcken, B., Green, K. & Rasko, J.E. Hartnup disorder: polymorphisms identified in the neutral amino acid transporter SLC1A5. J. Inherit. Metab. Dis. 25, 437–448 (2002).
Rasko, J.E.J., Battini, J.-L., Gottschalk, R.J., Mazo, I. & Miller, A.D. The RD114/simian type D retrovirus teceptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. USA 96, 2129–2134 (1999).
Avissar, N.E., Ryan, C.K., Ganapathy, V. & Sax, H.C. Na(+)-dependent neutral amino acid transporter ATB(0) is a rabbit epithelial cell brush-border protein. Am. J. Physiol. Cell Physiol. 281, C963–C971 (2001).
Verrey, F., Meier, C., Rossier, G. & Kuhn, L.C. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch. 440, 503–512 (2000).
Chillaron, J., Roca, R., Valencia, A., Zorzano, A. & Palacin, M. Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am. J. Physiol. Renal Physiol. 281, F995–F1018 (2001).
Wagner, C.A., Lang, F. & Broer, S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 281, C1077–C1093 (2001).
Kekuda, R. et al. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 271, 18657–18661 (1996).
Sloan, J.L. & Mager, S. Cloning and functional expression of a human Na(+) and Cl(−)-dependent neutral and cationic amino acid transporter B(0+). J. Biol. Chem. 274, 23740–23745 (1999).
Broer, A. et al. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to hartnup disorder. J. Biol. Chem. 279, 24467–24476 (2004).
Nozaki, J. et al. Homozygosity mapping to chromosome 5p15 of a gene responsible for Hartnup disorder. Biochem. Biophys. Res. Commun. 284, 255–260 (2001).
Wasserman, J.C., Delpire, E., Tonidandel, W., Kojima, R. & Gullans, S.R. Molecular characterization of ROSIT, a renal osmotic stress-induced Na(+)-Cl(−)-organic solute cotransporter. Am. J. Physiol. 267, F688–F694 (1994).
Nash, S.R. et al. Cloning, gene structure and genomic localization of an orphan transporter from mouse kidney with six alternatively-spliced isoforms. Receptors Channels 6, 113–128 (1998).
Wilcken, B., Smith, A. & Brown, D.A. Urine screening for aminoacidopathies: is it beneficial? Results of a long-term follow-up of cases detected by screening one million babies. J. Pediatrics 97, 492–497 (1980).
Pineda, M. et al. Cystinuria-specific rBAT(R365W) mutation reveals two translocation pathways in the amino acid transporter rBAT-b0, +AT. Biochem. J. 377, 665–674 (2004).
Ward, C.L. & Kopito, R.R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 269, 25710–25718 (1994).
Tarlow, M.J. et al. Absorption of amino acids and peptides in a child with a variant of Hartnup disease and coexistent coeliac disease. Arch. Dis. Child. 47, 798–803 (1972).
Scriver, C.R. et al. The Hartnup phenotype: Mendelian transport disorder, multifactorial disease. Am. J. Hum. Genet. 40, 401–412 (1987).
Broer, S. Xenopus laevis Oocytes. Methods Mol. Biol. 227, 245–258 (2003).
Rahman, B., Schneider, H.P., Broer, A., Deitmer, J.W. & Broer, S. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry 38, 11577–11584 (1999).
Kleta, R. et al. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat. Genet. advance online publication, 1 August 2004 (doi:10.1038/ng1405).
Acknowledgements
The Australian Hartnup Consortium represented in this publication thanks B. Wilcken for undertaking the original neonatal screening program in NSW that facilitated this study. We thank D.I.K. Martin and A. Basten for critical reading of the manuscript; the participating families for their generous consent; and C. Ng, J. Lai, O.T. Ooi and S. Kowalzcuk for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Hartnup disorder pedigrees. (PDF 46 kb)
Supplementary Table 1
Regions containing candidates studied by linkage analysis. (PDF 5 kb)
Rights and permissions
About this article
Cite this article
Seow, H., Bröer, S., Bröer, A. et al. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 36, 1003–1007 (2004). https://doi.org/10.1038/ng1406
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ng1406
This article is cited by
-
Structural insight into the substrate recognition and transport mechanism of amino acid transporter complex ACE2-B0AT1 and ACE2-SIT1
Cell Discovery (2023)
-
Dietary lysine level affects digestive enzyme, amino acid transport and hepatic intermediary metabolism in turbot (Scophthalmus maximus)
Fish Physiology and Biochemistry (2022)
-
COVID-19 and Hartnup disease: an affair of intestinal amino acid malabsorption
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2021)