Abstract
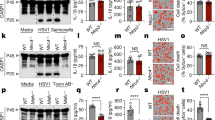
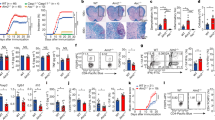
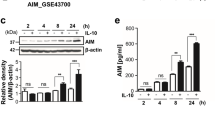
Inflammasomes regulate the activity of caspase-1 and the maturation of interleukin 1β (IL-1β) and IL-18. AIM2 has been shown to bind DNA and engage the caspase-1-activating adaptor protein ASC to form a caspase-1-activating inflammasome. Using Aim2-deficient mice, we identify a central role for AIM2 in regulating caspase-1-dependent maturation of IL-1β and IL-18, as well as pyroptosis, in response to synthetic double-stranded DNA. AIM2 was essential for inflammasome activation in response to Francisella tularensis, vaccinia virus and mouse cytomegalovirus and had a partial role in the sensing of Listeria monocytogenes. Moreover, production of IL-18 and natural killer cell–dependent production of interferon-γ, events critical in the early control of virus replication, were dependent on AIM2 during mouse cytomegalovirus infection in vivo. Collectively, our observations demonstrate the importance of AIM2 in the sensing of both bacterial and viral pathogens and in triggering innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kawai, T. & Akira, S. Toll-like receptor and RIG-I-like receptor signaling. Ann. NY Acad. Sci. 1143, 1–20 (2008).
Takeda, K. & Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 (2005).
Huysamen, C. & Brown, G.D. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol. Lett. 290, 121–128 (2009).
Yoneyama, M. & Fujita, T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 18, 545–551 (2007).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Chiu, Y.H., Macmillan, J.B. & Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III–transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature (2009).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Dinarello, C.A., Novick, D., Rubinstein, M. & Lonnemann, G. Interleukin 18 and interleukin 18 binding protein: possible role in immunosuppression of chronic renal failure. Blood Purif. 21, 258–270 (2003).
Boyden, E.D. & Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Latz, E. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22, 28–33 (2010).
Miao, E.A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA 107, 3076–3080 (2010).
Ludlow, L.E., Johnstone, R.W. & Clarke, C.J. The HIN-200 family: more than interferon-inducible genes? Exp. Cell Res. 308, 1–17 (2005).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11, 63–69 (2010).
Landolfo, S., Gariglio, M., Gribaudo, G. & Lembo, D. The Ifi 200 genes: an emerging family of IFN-inducible genes. Biochimie 80, 721–728 (1998).
Cole, L.E. et al. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J. Immunol. 180, 6885–6891 (2008).
Henry, T., Brotcke, A., Weiss, D.S., Thompson, L.J. & Monack, D.M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Elkins, K.L., Cowley, S.C. & Bosio, C.M. Innate and adaptive immunity to Francisella. Ann. NY Acad. Sci. 1105, 284–324 (2007).
Mariathasan, S., Weiss, D.S., Dixit, V.M. & Monack, D.M. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202, 1043–1049 (2005).
Cole, L.E. et al. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 75, 4127–4137 (2007).
Lamkanfi, M. & Dixit, V.M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Kanneganti, T.D. et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443 (2007).
Li, H., Nookala, S., Bina, X.R., Bina, J.E. & Re, F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J. Leukoc. Biol. 80, 766–773 (2006).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Meixenberger, K. et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1β, depending on listeriolysin O and NLRP3. J. Immunol. 184, 922–930 (2010).
Franchi, L., Kanneganti, T.D., Dubyak, G.R. & Nunez, G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 282, 18810–18818 (2007).
Warren, S.E., Mao, D.P., Rodriguez, A.E., Miao, E.A. & Aderem, A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J. Immunol. 180, 7558–7564 (2008).
Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 (1996).
Pien, G.C. & Biron, C.A. Compartmental differences in NK cell responsiveness to IL-12 during lymphocytic choriomeningitis virus infection. J. Immunol. 164, 994–1001 (2000).
Daniels, K.A. et al. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194, 29–44 (2001).
Hornung, V. & Latz, E. Intracellular DNA recognition. Nat. Rev. Immunol. 10, 123–130 (2010).
Ishikawa, H., Ma, Z. & Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Fitzgerald, K.A. et al. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Ichinohe, T., Lee, H.K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Labow, M. et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 159, 2452–2461 (1997).
Alcami, A. & Smith, G.L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell 71, 153–167 (1992).
Smith, G.L. & Chan, Y.S. Two vaccinia virus proteins structurally related to the interleukin-1 receptor and the immunoglobulin superfamily. J. Gen. Virol. 72, 511–518 (1991).
Johnston, J.B. et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598 (2005).
Henry, T. & Monack, D.M. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell. Microbiol. 9, 2543–2551 (2007).
Scalzo, A.A. et al. Genetic mapping of Cmv1 in the region of mouse chromosome 6 encoding the NK gene complex-associated loci Ly49 and musNKR-P1. Genomics 27, 435–441 (1995).
McVicar, D.W. et al. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J. Immunol. 169, 1721–1728 (2002).
Brown, M.G. et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292, 934–937 (2001).
Lee, S.H. et al. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28, 42–45 (2001).
Elkins, K.L., Winegar, R.K., Nacy, C.A. & Fortier, A.H. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb. Pathog. 13, 417–421 (1992).
Rathinam, V.A., Hoag, K.A. & Mansfield, L.S. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 10, 1316–1324 (2008).
Roberts, Z.J. et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J. Exp. Med. 204, 1559–1569 (2007).
Bukowski, J.F., Woda, B.A. & Welsh, R.M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52, 119–128 (1984).
Acknowledgements
We thank R. Barbalat (University of California at Berkeley) and G. Barton (University of California at Berkeley) for vaccinia virus; R. Welsh (University of Massachusetts Medical School) for mCMV; D. Knipe (Harvard Medical School) for HSV-1; M. O'Riordan (University of Michigan) for S. typhimurium; V. Boyartchuk (University of Massachusetts Medical School) for L. monocytogenes; T. Taniguchi (University of Tokyo) for Irf3−/− and Irf7−/− mice; P. Cresswell (Yale University) for antibody to viperin (anti-viperin); A. Cerny for animal husbandry and genotyping; A. Poltorak for guidance and help with genotyping mice; S. Schattgen for assistance with cell-viability assays; M. Pickering and T. Kowolik for discussions; E. Alnemri and T. Fernandez-Alnemri (Thomas Jefferson University) for discussions and polyclonal antibody to AIM2; and E. Lien for critical reading of the manuscript. Supported by the National Institutes of Health (AI083713 to K.A.F. and E.L.; CA66644 to E.T.S.; AI07349 to S.N.W.; CA034461 to R.W.; and U54 AI-157168 to S.N.V.) and the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (V.A.K.R.).
Author information
Authors and Affiliations
Contributions
K.A.F. oversaw the entire project; K.A.F., V.A.K.R. and S.N.W. conceived of the research with assistance from S.N.V., E.L. and E.S.-T.; V.A.K.R., B.G.M. and V.H. characterized the gene-trap integration; V.A.K.R. and L.W. did all the genotyping; V.A.K.R., Z.J. and S.N.W. designed and did the experiments with help from S.S., L.W., S.K.V. and S.G.; L.E.C. did the F. tularensis experiments; and K.A.F., V.A.K.R., S.S. and S.N.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 4782 kb)
Rights and permissions
About this article
Cite this article
Rathinam, V., Jiang, Z., Waggoner, S. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11, 395–402 (2010). https://doi.org/10.1038/ni.1864
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.1864
This article is cited by
-
A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis
Nature Communications (2024)
-
Caspase cleavage of RIPK3 after Asp333 is dispensable for mouse embryogenesis
Cell Death & Differentiation (2024)
-
Lactobacilli Probiotics Modulate Antibacterial Response Gene Transcription of Dendritic Cells Challenged with LPS
Probiotics and Antimicrobial Proteins (2024)
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Nuclear RPSA senses viral nucleic acids to promote the innate inflammatory response
Nature Communications (2023)