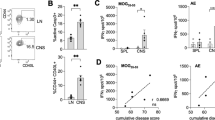
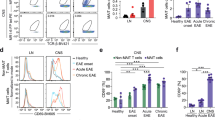
Abstract
Multiple sclerosis is an inflammatory, demyelinating, central nervous system disease mediated by myelin-specific T cells. Environmental triggers that cause the breakdown of myelin-specific T cell tolerance are unknown. Here we found that CD8+ myelin basic protein (MBP)-specific T cell tolerance was broken and autoimmunity was induced by infection with a virus that did not express MBP cross-reactive epitopes and did not depend on bystander activation. Instead, the virus activated T cells expressing dual T cell antigen receptors (TCRs) that were able to recognize both MBP and viral antigens. Our results demonstrate the importance of dual TCR–expressing T cells in autoimmunity and suggest a mechanism by which a ubiquitous viral infection could trigger autoimmunity in a subset of infected people, as suggested by the etiology of multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goverman, J., Perchellet, A. & Huseby, E.S. The role of CD8+ T cells in multiple sclerosis and its animal models. Curr. Drug Targets Inflamm. Allergy 4, 239–245 (2005).
Friese, M.A. & Fugger, L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain 128, 1747–1763 (2005).
Crawford, M.P. et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood 103, 4222–4231 (2004).
Coles, A.J. et al. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
De Jager, P.L. et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41, 776–782 (2009).
Cook, S.D. & Dowling, P.C. Multiple sclerosis and viruses: an overview. Neurology 30, 80–91 (1980).
Kurtzke, J.F. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 6, 382–427 (1993).
Cermelli, C. & Jacobson, S. Viruses and multiple sclerosis. Viral Immunol. 13, 255–267 (2000).
Gilden, D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 4, 195–202 (2005).
Vanderlugt, C.L. & Miller, S.D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2, 85–95 (2002).
Perchellet, A., Stromnes, I., Pang, J.M. & Goverman, J. CD8+ T cells maintain tolerance to myelin basic protein by 'epitope theft'. Nat. Immunol. 5, 606–614 (2004).
Goverman, J. et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72, 551–560 (1993).
Lafaille, J.J., Nagashima, K., Katsuki, M. & Tonegawa, S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell 78, 399–408 (1994).
Friese, M.A. et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat. Med. 14, 1227–1235 (2008).
Borgulya, P., Kishi, H., Uematsu, Y. & von Boehmer, H. Exclusion and inclusion of α and β T cell receptor alleles. Cell 69, 529–537 (1992).
Balomenos, D. et al. Incomplete T cell receptor Vβ allelic exclusion and dual Vβ-expressing cells. J. Immunol. 155, 3308–3312 (1995).
Hurst, S.D., Sitterding, S.M., Ji, S. & Barrett, T.A. Functional differentiation of T cells in the intestine of T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 94, 3920–3925 (1997).
Heath, W.R. & Miller, J.F. Expression of two α chains on the surface of T cells in T cell receptor transgenic mice. J. Exp. Med. 178, 1807–1811 (1993).
Munz, C., Lunemann, J.D., Getts, M.T. & Miller, S.D. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 9, 246–258 (2009).
Salvetti, M., Giovannoni, G. & Aloisi, F. Epstein-Barr virus and multiple sclerosis. Curr. Opin. Neurol. 22, 201–206 (2009).
Pohl, D. Epstein-Barr virus and multiple sclerosis. J. Neurol. Sci. 286, 62–64 (2009).
Ascherio, A. & Munger, K.L. Epstein-Barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. published online, doi:10.1007/s11481-010-9201-3 (6 April 2010).
De Jager, P.L. et al. Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology 70, 1113–1118 (2008).
Thacker, E.L., Mirzaei, F. & Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59, 499–503 (2006).
Nielsen, T.R. et al. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 64, 72–75 (2007).
Hosking, M.P. & Lane, T.E. The biology of persistent infection: inflammation and demyelination following murine coronavirus infection of the central nervous system. Curr Immunol Rev 5, 267–276 (2009).
Miller, S.D. et al. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3, 1133–1136 (1997).
Olson, J.K., Croxford, J.L., Calenoff, M.A., Dal Canto, M.C. & Miller, S.D. A virus-induced molecular mimicry model of multiple sclerosis. J. Clin. Invest. 108, 311–318 (2001).
Croxford, J.L., Ercolini, A.M., Degutes, M. & Miller, S.D. Structural requirements for initiation of cross-reactivity and CNS autoimmunity with a PLP139–151 mimic peptide derived from murine hepatitis virus. Eur. J. Immunol. 36, 2671–2680 (2006).
Tsunoda, I., Kuang, L.Q., Kobayashi-Warren, M. & Fujinami, R.S. Central nervous system pathology caused by autoreactive CD8+ T-cell clones following virus infection. J. Virol. 79, 14640–14646 (2005).
Mokhtarian, F., Zhang, Z., Shi, Y., Gonzales, E. & Sobel, R.A. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J. Neuroimmunol. 95, 43–54 (1999).
Cabbage, S.E. et al. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J. Immunol. 178, 887–896 (2007).
Teague, R.M. et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity 28, 662–674 (2008).
Alam, S.M. & Gascoigne, N.R. Posttranslational regulation of TCR Vα allelic exclusion during T cell differentiation. J. Immunol. 160, 3883–3890 (1998).
Elliott, J.I. & Altmann, D.M. Dual T cell receptor α chain T cells in autoimmunity. J. Exp. Med. 182, 953–959 (1995).
Heath, W.R. et al. Expression of two T cell receptor α chains on the surface of normal murine T cells. Eur. J. Immunol. 25, 1617–1623 (1995).
Padovan, E. et al. Expression of two T cell receptor α chains: dual receptor T cells. Science 262, 422–424 (1993).
Padovan, E. et al. Normal T lymphocytes can express two different T cell receptor β chains: implications for the mechanism of allelic exclusion. J. Exp. Med. 181, 1587–1591 (1995).
Davodeau, F. et al. Dual T cell receptor β chain expression on human T lymphocytes. J. Exp. Med. 181, 1391–1398 (1995).
He, X. et al. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat. Immunol. 3, 127–134 (2002).
Padovan, E., Casorati, G., Dellabona, P., Giachino, C. & Lanzavecchia, A. Dual receptor T-cells. Implications for alloreactivity and autoimmunity. Ann. NY Acad. Sci. 756, 66–70 (1995).
Morris, G.P. & Allen, P.M. Cutting edge: Highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. J. Immunol. 182, 6639–6643 (2009).
Elliott, E.A. et al. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J. Clin. Invest. 98, 1602–1612 (1996).
Corthay, A., Nandakumar, K.S. & Holmdahl, R. Evaluation of the percentage of peripheral T cells with two different T cell receptor α-chains and of their potential role in autoimmunity. J. Autoimmun. 16, 423–429 (2001).
Brabb, T. et al. Triggers of autoimmune disease in a murine T-cell receptor transgenic model for multiple sclerosis. J. Immunol. 159, 497–507 (1997).
Olivares-Villagomez, D., Wang, Y. & Lafaille, J.J. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188, 1883–1894 (1998).
Serafini, B. et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 204, 2899–2912 (2007).
Willis, S.N. et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 132, 3318–3328 (2009).
Stromnes, I.M., Cerretti, L.M., Liggitt, D., Harris, R.A. & Goverman, J.M. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nat. Med. 14, 337–342 (2008).
Harrington, C.J. et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity 8, 571–580 (1998).
Acknowledgements
We thank N. Mausolf for animal husbandry and technical assistance and S. Lee and E. Pierson for critical comments on the manuscript. Supported by the National Institutes of Health (AI07272737 to J.M.G.).
Author information
Authors and Affiliations
Contributions
Q.J. did most of the experiments and analyzed the data; A.P. did the initial disease-induction experiments and critiqued the manuscript; Q.J. and J.M.G. designed the study and wrote the manuscript; and J.M.G. secured the funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Table 1 (PDF 3398 kb)
Supplementary Video 1
EAE in 8.8 mice after wild-type vaccinia infection. Female 8.8 mice were intraperitoneally infected with 5×106 pfu of wild-type vaccinia virus. This video was taken one month after vaccinia infection. (MOV 2886 kb)
Rights and permissions
About this article
Cite this article
Ji, Q., Perchellet, A. & Goverman, J. Viral infection triggers central nervous system autoimmunity via activation of CD8+ T cells expressing dual TCRs. Nat Immunol 11, 628–634 (2010). https://doi.org/10.1038/ni.1888
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.1888
This article is cited by
-
scRNA-seq revealed the special TCR β & α V(D)J allelic inclusion rearrangement and the high proportion dual (or more) TCR-expressing cells
Cell Death & Disease (2023)
-
Development of TCR-T cell therapy targeting mismatched HLA-DPB1 for relapsed leukemia after allogeneic transplantation
International Journal of Hematology (2023)
-
De- “bug”-ing the microbiome in lung cancer
Cancer and Metastasis Reviews (2022)
-
CD8+ T cells specific for cryptic apoptosis-associated epitopes exacerbate experimental autoimmune encephalomyelitis
Cell Death & Disease (2021)
-
Heterotypic immunity against vaccinia virus in an HLA-B*07:02 transgenic mousepox infection model
Scientific Reports (2020)