Abstract
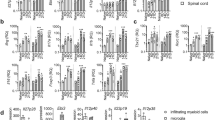
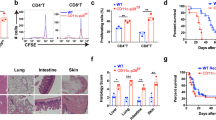
The heterodimeric cytokine interleukin 27 (IL-27) signals through the IL-27Rα subunit of its receptor, combined with gp130, a common receptor chain used by several cytokines, including IL-6. Notably, the IL-27 subunits p28 (IL-27p28) and EBI3 are not always expressed together, which suggests that they may have unique functions. Here we show that IL-27p28, independently of EBI3, antagonized cytokine signaling through gp130 and IL-6-mediated production of IL-17 and IL-10. Similarly, the ability to generate antibody responses was dependent on the activity of gp130-signaling cytokines. Mice transgenic for expression of IL-27p28 showed a substantial defect in the formation of germinal centers and antibody production. Thus, IL-27p28, as a natural antagonist of gp130-mediated signaling, may be useful as a therapeutic for managing inflammation mediated by cytokines that signal through gp130.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
21 January 2011
In the version of this article initially published, the author name M. Merle Elloso and the associated affiliation were incorrect. The correct affiliation is Centocor Research and Development, Inc. The error has been corrected in the HTML and PDF versions of the article.
References
Kastelein, R.A., Hunter, C.A. & Cua, D.J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242 (2007).
Damsker, J.M., Hansen, A.M. & Caspi, R.R. Th1 and Th17 cells: adversaries and collaborators. Ann. NY Acad. Sci. 1183, 211–221 (2010).
Jones, S.A. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 (2005).
Jones, S.A., Richards, P.J., Scheller, J. & Rose-John, S. IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 25, 241–253 (2005).
Ding, C., Cicuttini, F., Li, J. & Jones, G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin. Investig. Drugs 18, 1457–1466 (2009).
Benigni, F. et al. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood 87, 1851–1854 (1996).
Hangoc, G. et al. In vivo effects of recombinant interleukin-11 on myelopoiesis in mice. Blood 81, 965–972 (1993).
Metcalf, D., Nicola, N.A. & Gearing, D.P. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood 76, 50–56 (1990).
Muraguchi, A. et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J. Exp. Med. 167, 332–344 (1988).
Muraguchi, A. et al. T cell-replacing factor- (TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J. Immunol. 127, 412–416 (1981).
Senaldi, G. et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc. Natl. Acad. Sci. USA 96, 11458–11463 (1999).
Larousserie, F. et al. Differential effects of IL-27 on human B cell subsets. J. Immunol. 176, 5890–5897 (2006).
Pflanz, S. et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16, 779–790 (2002).
Pflanz, S. et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 (2004).
Heinzel, F.P., Hujer, A.M., Ahmed, F.N. & Rerko, R.M. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 158, 4381–4388 (1997).
Batten, M. & Ghilardi, N. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 85, 661–672 (2007).
Devergne, O. et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J. Virol. 70, 1143–1153 (1996).
Maaser, C., Egan, L.J., Birkenbach, M.P., Eckmann, L. & Kagnoff, M.F. Expression of Epstein-Barr virus-induced gene 3 and other interleukin-12-related molecules by human intestinal epithelium. Immunology 112, 437–445 (2004).
Sonobe, Y. et al. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 1040, 202–207 (2005).
Collison, L.W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Niedbala, W. et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 37, 3021–3029 (2007).
Devergne, O., Birkenbach, M. & Kieff, E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. USA 94, 12041–12046 (1997).
Stumhofer, J.S. et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7, 937–945 (2006).
Kumanogoh, A. et al. Impairment of antigen-specific antibody production in transgenic mice expressing a dominant-negative form of gp130. Proc. Natl. Acad. Sci. USA 94, 2478–2482 (1997).
Senaldi, G. et al. Regulatory effects of novel neurotrophin-1/B cell-stimulating factor-3 (cardiotrophin-like cytokine) on B cell function. J. Immunol. 168, 5690–5698 (2002).
Takatsuki, F. et al. Human recombinant IL-6/B cell stimulatory factor 2 augments murine antigen-specific antibody responses in vitro and in vivo. J. Immunol. 141, 3072–3077 (1988).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Shimozato, O. et al. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology 128, e816–e825 (2009).
Liu, J., Guan, X. & Ma, X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-γ-mediated pathways. J. Exp. Med. 204, 141–152 (2007).
Molle, C. et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J. Immunol. 178, 7607–7615 (2007).
Stumhofer, J.S. et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 (2007).
McGeachy, M.J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Fischer, M. et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 (1997).
Timmermann, A. et al. Different epitopes are required for gp130 activation by interleukin-6, oncostatin M and leukemia inhibitory factor. FEBS Lett. 468, 120–124 (2000).
Iritani, B.M., Forbush, K.A., Farrar, M.A. & Perlmutter, R.M. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 16, 7019–7031 (1997).
Jack, R.S., Imanishi-Kari, T. & Rajewsky, K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur. J. Immunol. 7, 559–565 (1977).
Arend, W.P., Palmer, G. & Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223, 20–38 (2008).
Mentink-Kane, M.M. et al. IL-13 receptor α2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc. Natl. Acad. Sci. USA 101, 586–590 (2004).
Crabe, S. et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J. Immunol. 183, 7692–7702 (2009).
Scheller, J., Schuster, B., Holscher, C., Yoshimoto, T. & Rose-John, S. No inhibition of IL-27 signaling by soluble gp130. Biochem. Biophys. Res. Commun. 326, 724–728 (2005).
Hibi, M. et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63, 1149–1157 (1990).
Brakenhoff, J.P., Bos, H.K., Grotzinger, J., Rose-John, S. & Aarden, L.A. Identification of residues in the putative 5th helical region of human interleukin-6, important for activation of the IL-6 signal transducer, gp130. FEBS Lett. 395, 235–240 (1996).
Schroers, A. et al. Dynamics of the gp130 cytokine complex: a model for assembly on the cellular membrane. Protein Sci. 14, 783–790 (2005).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Wu, Y. et al. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int. Immunol. 21, 745–756 (2009).
Fattori, E. et al. Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood 83, 2570–2579 (1994).
Shen, M.M. et al. Expression of LIF in transgenic mice results in altered thymic epithelium and apparent interconversion of thymic and lymph node morphologies. EMBO J. 13, 1375–1385 (1994).
Chae, S.C. et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J. Hum. Genet. 52, 355–361 (2007).
Imielinski, M. et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 41, 1335–1340 (2009).
Li, C.S. et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J. Gastroenterol. Hepatol. 24, 1692–1696 (2009).
O'Shea, J. J. Targeting the Jak/STAT pathway for immunosuppression. Ann. Rheum. Dis. 63, ii67–ii71 (2004).
O'Shea, J.J., Pesu, M., Borie, D.C. & Changelian, P.S. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 3, 555–564 (2004).
Howlett, M., Menheniott, T.R., Judd, L.M. & Giraud, A.S. Cytokine signalling via gp130 in gastric cancer. Biochim. Biophys. Acta 1793, 1623–1633 (2009).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Hogan, B., Beddington, R. Costantini, F. & Lacy, E. Manipulating the Mouse Embryo 2nd edn. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA, 1994).
Vriend, G. WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 8, 52–56 (1990).
Carson, M. Ribbons. Methods Enzymol. 277, 493–505 (1997).
Allman, D.M., Ferguson, S.E., Lentz, V.M. & Cancro, M.P. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 151, 4431–4444 (1993).
Scholz, J.L. et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl. Acad. Sci. USA 105, 15517–15522 (2008).
Acknowledgements
We thank D. Gorman for generating the IL-27 and IL-27p28 minicircles. Supported by the US National Institutes for Health (AI-042334 to C.A.H.; AI-054488 to M.P.C.; 1-T32-AI-055428 to J.S.S. and W.J.Q. III; and 2-T32-AI-007532-11 to E.D.T.), the Abramson Cancer Center (Center for Digestive Diseases), the state of Pennsylvania, the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB415, B5 to S.R-J., and SFB415, B7 to J.G. and B.S.), the Cluster of Excellence 'Inflammation at Interfaces' and the Marie Lowe Cancer Center of the University of Pennsylvania (C.A.H.).
Author information
Authors and Affiliations
Contributions
J.S.S. and C.A.H. contributed to all studies and wrote the manuscript; E.D.T., W.J.Q. III, N.H., M.P.C. and S.D.L. were involved in analyzing p28-transgenic mice; R.G. contributed to studies of GC formation; C.J.M.S. contributed to the studies of Il27ra−/− mice; M.M.E. contributed to studies with Ebi3−/− mice; A.C.O. contributed to studies of intracellular staining for IL-27p28; B.S., S.R.-J. and J.G. did the p28-gp130 modeling and contributed to its analysis; C.A.F. and S.A.J. did the biacore assays and contributed to their analysis; M.L.J. provided the recombinant IL-27p28 protein; and Y.C. and D.J.C. did hydrodynamics-based transfection experiments with minicircle DNA and contributed to their analysis.
Corresponding author
Ethics declarations
Competing interests
C.A.H. and J.S.S. have a patent application on the use of p28 to limit gp130 signaling. N.H. and S.D.L. are employees of ZymoGenetics; Y.C. and D.J.C. are employees of DNAX Discovery Research; M.L.J. is an employee of Shenandoah Biotechnology; C.J.M.S. is an employee of Amgen; and M.M.E. is an employee of Centocor Research and Development.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 1207 kb)
Rights and permissions
About this article
Cite this article
Stumhofer, J., Tait, E., III, W. et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol 11, 1119–1126 (2010). https://doi.org/10.1038/ni.1957
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.1957
This article is cited by
-
Cell type specific IL-27p28 (IL-30) deletion in mice uncovers an unexpected regulatory function of IL-30 in autoimmune inflammation
Scientific Reports (2023)
-
Systemic IL-27 administration prevents abscess formation and osteolysis via local neutrophil recruitment and activation
Bone Research (2022)
-
Dendritic cell-derived IL-27 p28 regulates T cell program in pathogenicity and alleviates acute graft-versus-host disease
Signal Transduction and Targeted Therapy (2022)
-
IL-30† (IL-27A): a familiar stranger in immunity, inflammation, and cancer
Experimental & Molecular Medicine (2021)
-
The clinical significance of IL-6 s and IL-27 s in Bronchoalveolar lavage fluids from children with mycoplasma pneumoniae pneumonia
BMC Infectious Diseases (2020)