Abstract
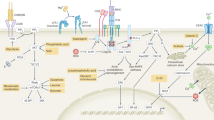
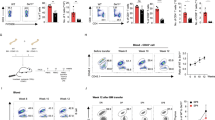
The kinase mTOR has emerged as an important regulator of the differentiation of helper T cells. Here we demonstrate that differentiation into the TH1 and TH17 subsets of helper T cells was selectively regulated by signaling from mTOR complex 1 (mTORC1) that was dependent on the small GTPase Rheb. Rheb-deficient T cells failed to generate TH1 and TH17 responses in vitro and in vivo and did not induce classical experimental autoimmune encephalomyelitis (EAE). However, they retained their ability to become TH2 cells. Alternatively, when mTORC2 signaling was deleted from T cells, they failed to generate TH2 cells in vitro and in vivo but preserved their ability to become TH1 and TH17 cells. Our data identify mechanisms by which two distinct signaling pathways downstream of mTOR regulate helper cell fate in different ways. These findings define a previously unknown paradigm that links T cell differentiation with selective metabolic signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
06 May 2011
In the version of this article initially published, the Discussion section cited a published study of mice with conditional deletion of Rictor in T cells without specifying which Lck promoter was used to drive the expression of Cre recombinase. The distal Lck promoter (dLck) was used. The error has been corrected in the HTML and PDF versions of the article.
References
Guertin, D.A. & Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 (2007).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Kim, D.H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Sarbassov, D.D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Laplante, M. & Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 (2009).
Holz, M.K. & Blenis, J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J. Biol. Chem. 280, 26089–26093 (2005).
Guertin, D.A. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell 11, 859–871 (2006).
Delgoffe, G.M. & Powell, J.D. mTOR: taking cues from the immune microenvironment. Immunology 127, 459–465 (2009).
Delgoffe, G.M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Rao, R.R., Li, Q., Odunsi, K. & Shrikant, P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity 32, 67–78 (2010).
Sinclair, L.V. et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat. Immunol. 9, 513–521 (2008).
Yamagata, K. et al. Rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J. Biol. Chem. 269, 16333–16339 (1994).
Yee, W.M. & Worley, P.F. Rheb interacts with Raf-1 kinase and may function to integrate growth factor- and protein kinase A-dependent signals. Mol. Cell. Biol. 17, 921–933 (1997).
Manning, B.D. & Cantley, L.C. Rheb fills a GAP between TSC and TOR. Trends Biochem. Sci. 28, 573–576 (2003).
Saucedo, L.J. et al. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 5, 566–571 (2003).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Wensky, A.K. et al. IFN-γ determines distinct clinical outcomes in autoimmune encephalomyelitis. J. Immunol. 174, 1416–1423 (2005).
Kumar, A. et al. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances basal glycogen synthase activity. Mol. Cell. Biol. 28, 61–70 (2008).
Paul, W.E. What determines Th2 differentiation, in vitro and in vivo? Immunol. Cell Biol. 88, 236–239 (2010).
Yamane, H., Zhu, J. & Paul, W.E. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 202, 793–804 (2005).
Sarbassov, D.D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Wang, B. et al. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS ONE 3, e1856 (2008).
Zhou, J. et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA 104, 16158–16163 (2007).
Yamamoto, K., Yamaguchi, M., Miyasaka, N. & Miura, O. SOCS-3 inhibits IL-12-induced STAT4 activation by binding through its SH2 domain to the STAT4 docking site in the IL-12 receptor β2 subunit. Biochem. Biophys. Res. Commun. 310, 1188–1193 (2003).
Yu, C.R. et al. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J. Biol. Chem. 278, 29752–29759 (2003).
Palmer, D.C. & Restifo, N.P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30, 592–602 (2009).
Ozaki, A., Seki, Y., Fukushima, A. & Kubo, M. The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J. Immunol. 175, 5489–5497 (2005).
Seki, Y. et al. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl. Acad. Sci. USA 99, 13003–13008 (2002).
Battaglia, M., Stabilini, A. & Roncarolo, M.G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 105, 7797–7802 (2008).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Ballou, L.M., Selinger, E.S., Choi, J.Y., Drueckhammer, D.G. & Lin, R.Z. Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl)pyrimido[2,1-α]isoquinolin-4-one. J. Biol. Chem. 282, 24463–24470 (2007).
Colombetti, S., Basso, V., Mueller, D.L. & Mondino, A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J. Immunol. 176, 2730–2738 (2006).
Rathmell, J.C., Farkash, E.A., Gao, W. & Thompson, C.B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876 (2001).
Stephenson, L.M., Park, D.S., Mora, A.L., Goenka, S. & Boothby, M. Sequence motifs in IL-4Rα mediating cell-cycle progression of primary lymphocytes. J. Immunol. 175, 5178–5185 (2005).
Lekmine, F. et al. Interferon-gamma engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp. Cell Res. 295, 173–182 (2004).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Kang, J., Huddleston, S.J., Fraser, J.M. & Khoruts, A. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J. Leukoc. Biol. 83, 1230–1239 (2008).
Kopf, H., de la Rosa, G.M., Howard, O.M. & Chen, X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int. Immunopharmacol. 7, 1819–1824 (2007).
Valmori, D. et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. 177, 944–949 (2006).
Francisco, L.M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Cobbold, S.P. et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci. USA 106, 12055–12060 (2009).
Kim, S. et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron 66, 69–84 (2010).
Shu, L. & Houghton, P.J. The mTORC2 complex regulates terminal differentiation of C2C12 myoblasts. Mol. Cell. Biol. 29, 4691–4700 (2009).
Johnson, M.D., O'Connell, M., Vito, F. & Bakos, R.S. Increased STAT-3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J. Neurooncol. 92, 129–136 (2009).
Garcia-Martinez, J.M. & Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 416, 375–385 (2008).
Dekker, R.J. et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood 107, 4354–4363 (2006).
Delgoffe, G.M., Kole, T.P., Cotter, R.J. & Powell, J.D. Enhanced interaction between Hsp90 and raptor regulates mTOR signaling upon T cell activation. Mol. Immunol. 46, 2694–2698 (2009).
Acknowledgements
We thank P.F. Worley (Johns Hopkins University) for mice with loxP-flanked Rheb alleles; M. Magnuson (Vanderbilt University) for mice with loxP-flanked Rictor alleles; S.C. Kozma (University of Cincinnati) for mice with loxP-flanked Mtor alleles; C. Drake (Johns Hopkins University) for vaccinia virus expressing ovalbumin; members of the Powell laboratory; and C. Drake and D. Pardoll for discussions and reagents. Supported by the US National Institutes of Health (R01AI077610-01A2).
Author information
Authors and Affiliations
Contributions
G.M.D. did research, helped design experiments and wrote the paper; K.N.P. assisted with in vivo experiments and biochemistry; A.T.W. assisted with EAE induction and central nervous system isolation and did immunohistochemistry; E.H. assisted with very-low-dose rapamycin experiments; D.J.M. synthesized the mTOR kinase inhibitor; M.R.H. helped design experiments and contributed reagents; B.X. and P.F.W. generated the original mouse line with loxP-flanked Rheb alleles; and J.D.P. designed experiments, oversaw research and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 9152 kb)
Supplementary Video 1
Rheb-deficient T cells induce nonclassical EAE. Video of a representative T-Rheb−/− mouse 14 days after immunization with MOG peptide + CFA to induce EAE. The mouse displays symptoms of ataxia without paralysis. (MOV 5111 kb)
Rights and permissions
About this article
Cite this article
Delgoffe, G., Pollizzi, K., Waickman, A. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12, 295–303 (2011). https://doi.org/10.1038/ni.2005
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2005
This article is cited by
-
Mechanistic target of rapamycin (mTOR): a potential new therapeutic target for rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
The SMYD3-dependent H3K4me3 status of IGF2 intensifies local Th2 differentiation in CRSwNP via positive feedback
Cell Communication and Signaling (2023)
-
Tumor-secreted IFI35 promotes proliferation and cytotoxic activity of CD8+ T cells through PI3K/AKT/mTOR signaling pathway in colorectal cancer
Journal of Biomedical Science (2023)
-
mTORC2 acts as a gatekeeper for mTORC1 deficiency-mediated impairments in ILC3 development
Acta Pharmacologica Sinica (2023)
-
Rise of the natural red pigment ‘prodigiosin’ as an immunomodulator in cancer
Cancer Cell International (2022)